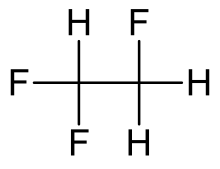
1,1,2-Trifluoroethane
 | |
| Names | |
|---|---|
| Other names
HFC-143, R-143, asymmetrical trifluoroethane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.006.425 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H3F3 | |
| Molar mass | 84.041 g·mol−1 |
| Appearance | colourless gas |
| Melting point | −84 °C (−119 °F; 189 K) [1] |
| Boiling point | 5 °C (41 °F; 278 K) [1] |
| Related compounds | |
Related compounds
|
Trifluoroethylene; 1,1,1-trifluoroethane; 1,1,2-Trichloroethane; 1,1,2-Tribromoethane; 1,1,2-Triiodoethane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
1,1,2-Trifluoroethane or R-143, is a hydrofluorocarbon with formula CH2FCHF2. It is a colourless gas at room temperature. It is an asymmetrical isomer of 1,1,1-trifluoroethane. 1,1,2-Trifluoroethane has a global warming potential of 397 for 100 years.[2]
1,1,2-Trifluoroethane can be obtained by the hydrogenation of 1,2-dichlorodifluoroethylene or chlorotrifluoroethylene.
See also
References
- ^ a b Chemspider entry
- ^ G. Myhre, D. Shindell et al.: Climate Change 2013: The Physical Science Basis. Working Group I contribution to the IPCC Fifth Assessment Report. Hrsg.: Intergovernmental Panel on Climate Change. 2013, Chapter 8: Anthropogenic and Natural Radiative Forcing, 24–39; Table 8.SM.16