1,2-Epoxybutane
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Ethyloxirane | |
| Other names
1,2-Butyleneoxide
1,2-Buteneoxide Ethyloxyrane | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.003.127 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3022 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H8O | |
| Molar mass | 72.107 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.83 g·cm−3 |
| Melting point | −150 °C (−238 °F; 123 K) |
| Boiling point | 65 °C (149 °F; 338 K) |
| Viscosity | 0.40 mPa.s |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H225, H302, H312, H315, H319, H332, H335, H351, H412 | |
| P201, P202, P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P273, P280, P281, P301+P312, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P308+P313, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P370+P378, P403+P233, P403+P235, P405, P501 | |
| Flash point | −22 °C (−8 °F; 251 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
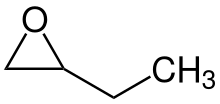
1,2-Epoxybutane is an organic compound with the formula CH2(O)CHCH2CH3. It is a chiral epoxide prepared by oxidation of 1-butene.[1]
References
- ^ Kamata, Keigo; Yonehara, Koji; Sumida, Yasutaka; Yamaguchi, Kazuya; Hikichi, Shiro; Mizuno, Noritaka (2003). "Efficient Epoxidation of Olefins with ≥99% Selectivity and Use of Hydrogen Peroxide". Science. 300 (5621): 964–966. Bibcode:2003Sci...300..964K. doi:10.1126/science.1083176. PMID 12738860. S2CID 38700647.