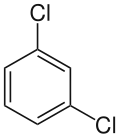
1,3-Dichlorobenzene
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,3-Dichlorobenzene | |
| Other names
m-Dichlorobenzene; meta-Dichlorobenzene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.007.994 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H4Cl2 | |
| Molar mass | 147.00 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.288 g/cm3 |
| Melting point | −22 to −25 °C (−8 to −13 °F; 251 to 248 K) |
| Boiling point | 172 to 173 °C (342 to 343 °F; 445 to 446 K) |
| Insoluble | |
| −83.19·10−6 cm3/mol | |
| Hazards | |
| Flash point | 65 °C (149 °F; 338 K) |
| Related compounds | |
Related compounds
|
1,3-Dibromobenzene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
1,3-Dichlorobenzene (also known as meta-dichlorobenzene) is an aryl chloride and isomer of dichlorobenzene with the formula C6H4Cl2. It is the least common of the three isomers of dichlorobenzene, and it is a colorless liquid that is insoluble in water. It is produced as a minor byproduct of the chlorination of benzene, but can also be prepared in a directed manner by the Sandmeyer reaction of 3-chloroaniline. It also arises from the isomerization of the other dichlorobenzenes at high temperature.[1]
Hazards
This chemical is combustible. "Hazardous decomposition products" are carbon monoxide, carbon dioxide, chlorine, hydrogen chloride gas. It is toxic to aquatic life with long-lasting effects.[2]
References
- ^ U. Beck, E. Löser "Chlorinated Benzenes and other Nucleus-Chlorinated Aromatic Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry, 2012, Wiley-VCH, Weinheim. doi:10.1002/14356007.o06_o03
- ^ "SAFETY DATA SHEET". Thermo Fisher Scientific. Retrieved 9 February 2021.