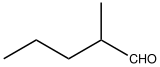
2-Methylpentanal
 | |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.188 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 2367 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H12O | |
| Molar mass | 100.161 g·mol−1 |
| Appearance | colorless liqiud |
| Density | 0.86 g/cm3 |
| Boiling point | 137–138 °C (279–280 °F; 410–411 K) |
Refractive index (nD)
|
1.45 |
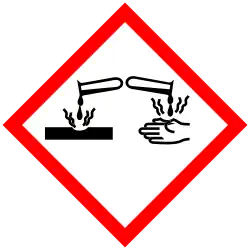
| Hazards | |
| GHS labelling:[1] | |
| |
| Danger | |
| H225, H315, H319 | |
| P210, P233, P240, P241, P242, P243, P264, P264+P265, P273, P280, P302+P352, P303+P361+P353, P305+P351+P338, P321, P332+P317, P337+P317, P362+P364, P370+P378, P403+P235, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
2-Methylpentanal is an organic compound with the formula CH3CH2CH2CH(CH3)CHO. It is a colorless liquid. The compound is a chiral aldehyde. It serves as a precursor to the commercial tranquilizer meprobamate. It is prepared by aldol condensation of propionaldehyde followed by dehydration and hydrogenation of the aldol product.[1]
References
- ^ Kohlpaintner, Christian; Schulte, Markus; Falbe, Jürgen; Lappe, Peter; Weber, Jürgen (2008). "Aldehydes, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a01_321.pub2. ISBN 978-3-527-30673-2.