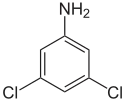
3,5-Dichloroaniline
 | |
| Names | |
|---|---|
| Other names
1-amino-3,5- dichlorobenzene
| |
| Identifiers | |
3D model (JSmol)
|
|
| 636492 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.009.954 |
| EC Number |
|
| 363409 | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H5Cl2N | |
| Molar mass | 162.01 g·mol−1 |
| Appearance | colorless solid |
| Density | 1.58 g/cm3 |
| Melting point | 51–53 °C (124–127 °F; 324–326 K) |
| Boiling point | 260 °C (500 °F; 533 K) 741 torr |
| Hazards | |
| GHS labelling:[1] | |
| |
| Danger | |
| H301, H311, H331, H373, H410 | |
| P260, P261, P262, P264, P270, P271, P273, P280, P301+P316, P302+P352, P304+P340, P316, P319, P321, P330, P361+P364, P391, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
3,5-Dichloroaniline is an organic compound with the formula C6H3Cl2(NH2). It is one of several isomers of dichloroaniline. It is a colorless solid although commercial samples often appear colored. It is produced by hydrogenation of 3,5-dichloronitrobenzene.[2] It is a precursor to the fungicide vinclozolin.
Safety and environmental aspects
Its 72-h EC50 in an algal growth inhibition assay is 4.39 mg/L.[3] Biodegradation of dichloroanilines usually proceeds via initial ring hydroxylation.[4]
References
- ^ "3,5-Dichloroaniline". pubchem.ncbi.nlm.nih.gov.
- ^ Jagadeesh, Rajenahally V.; Surkus, Annette-Enrica; Junge, Henrik; Pohl, Marga-Martina; Radnik, Jörg; Rabeah, Jabor; Huan, Heming; Schünemann, Volker; Brückner, Angelika; Beller, Matthias (2013). "Nanoscale Fe2O3-Based Catalysts for Selective Hydrogenation of Nitroarenes to Anilines". Science. 342 (6162): 1073–1076. Bibcode:2013Sci...342.1073J. doi:10.1126/science.1242005. PMID 24288327.
- ^ Aruoja, Villem; Sihtmäe, Mariliis; Dubourguier, Henri-Charles; Kahru, Anne (2011). "Toxicity of 58 substituted anilines and phenols to algae Pseudokirchneriella subcapitata and bacteria Vibrio fischeri: Comparison with published data and QSARs". Chemosphere. 84 (10): 1310–1320. Bibcode:2011Chmsp..84.1310A. doi:10.1016/j.chemosphere.2011.05.023. PMID 21664645.
- ^ Suchana, Shamsunnahar; Araujo, Sofia Pimentel; Lomheim, Line; Mack, E. Erin; Spain, Jim C.; Edwards, Elizabeth; Passeport, Elodie (2024). "Compound-Specific Carbon, Nitrogen, and Hydrogen Isotope Analysis to Characterize Aerobic Biodegradation of 2,3-Dichloroaniline by a Mixed Enrichment Culture". Environmental Science & Technology. 58 (27): 12042–12050. Bibcode:2024EnST...5812042S. doi:10.1021/acs.est.4c02173. PMID 38934904.