3-Hydroxyaspartic acid
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(2S)-2-Amino-3-hydroxybutanedioic acid | |
| Other names
(2S)-2-Amino-3-hydroxysuccinic acid
3-Hydroxyaspartic acid Beta-hydroxyaspartic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H7NO5 | |
| Molar mass | 149.102 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
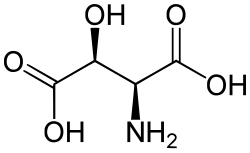
3-Hydroxyaspartic acid (three letter abbreviation: Hya) also known as beta-hydroxyaspartic acid is derivative of aspartic acid which has been hydroxylated at position-3. The adjacent image shows L-threo-3-Hydroxyaspartate. The conjugated acid of 3-hydroxyaspartic acid is 3-hydroxyaspartate.
Structure
Similarly to proteinogenic isoleucine and threonine, 3-hydroxyaspartic acid contains two chiral centers. As such, it can exist in 4 stereoisomers, which form two pairs of enantiomers.
Function
The Hya amino acid residue is sometimes contained in EGF-like domains such as Vitamin K-dependent coagulation plasma proteins including protein C.[1]
D-threo-3-Hydroxyaspartate is a part of the siderophore ornibactin.[2]
See also
References
- ^ Castellino FJ, Ploplis VA, Zhang L (2008). "Γ-Glutamate and β–Hydroxyaspartate in Proteins". gamma-Glutamate and beta-hydroxyaspartate in proteins. Methods Mol. Biol. Vol. 446. pp. 85–94. doi:10.1007/978-1-60327-084-7_6. ISBN 978-1-58829-719-8. PMID 18373251.
- ^ Stephan, Holger; Freund, Stefan; Beck, Werner; Jung, Günther; Meyer, Jean-Marie; Winkelmann, Günther (1993-06-01). "Ornibactins—a new family of siderophores from Pseudomonas". Biometals. 6 (2): 93–100. doi:10.1007/BF00140109. ISSN 1572-8773.