4-Piperidone
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Piperidin-4-one | |
| Other names
4-Piperidone
Azinanone Azinan-4-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.050.420 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H9NO | |
| Molar mass | 99.133 g·mol−1 |
| Boiling point | 79 °C (174 °F; 352 K) |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 91 °C (196 °F; 364 K) |
| Related compounds | |
Related compounds
|
Piperidine; 2-Piperidinone |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
4-Piperidone is an organic compound with the molecular formula OC(CH2)4NH. It can be viewed as a derivative of piperidine. 4-Piperidone is used as an intermediate in the manufacture of chemicals and pharmaceutical drugs. Substituted and dehydro derivatives of 4-piperidinone are intermediates in alkaloid syntheses.[1]
The N-protonated derivative is typically isolated as the hydrate (HO)2C(CH2)4NH+2.[2]
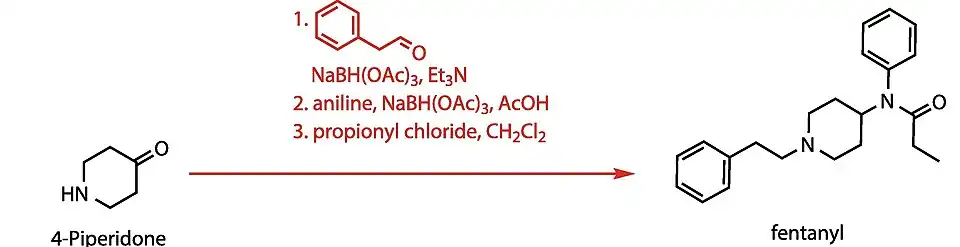
It is a List I chemical in the United States as it is a precursor to fentanyl.
See also
References
- ^ Weintraub, Philip M.; Sabol, Jeffrey S.; Kane, John M.; Borcherding, David R. (2003). "Recent advances in the synthesis of piperidones and piperidines". Tetrahedron. 59 (17): 2953–2989. doi:10.1016/s0040-4020(03)00295-3.
- ^ Gamrad, Waltraud; Dreier, Angelika; Goddard, Richard; Pörschke, Klaus-Richard (2015). "Cation-Cation Pairing by N-C-H⋅⋅⋅O Hydrogen Bonds". Angewandte Chemie International Edition. 54 (15): 4482–4487. doi:10.1002/anie.201408278. PMID 25712229.
