Alixorexton
 | |
| Clinical data | |
|---|---|
| Other names | ALKS-2680; ALKS2680 |
| Routes of administration | Oral |
| Drug class | Orexin OX2 receptor agonist |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| UNII | |
| Chemical and physical data | |
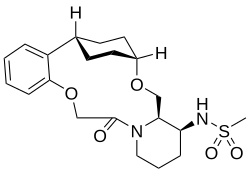
| Formula | C21H30N2O5S |
| Molar mass | 422.54 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Alixorexton (INN; developmental code name ALKS-2680) is an orexin receptor agonist which is under development for the treatment of sleep disorders, including narcolepsy and idiopathic hypersomnia.[1][2][3] Alixorexton is being developed by Alkermes[4] As of July 2025, it is in phase 2 clinical trials with planned advancement to a phase 3 study for the treatment of narcolepsy type 1.[4]
See also
References
- ^ "Alixorexton - Alkermes". AdisInsight. Springer Nature Switzerland AG. Retrieved 2025-07-30.
- ^ Morse AM (May 2025). "Enhancing the Management of Hypersomnia: Examining the Role of the Orexin System". Seminars in Neurology. 45 (3): 410–419. doi:10.1055/a-2589-3825. PMID 40239951.
- ^ Prakash BA, Shah I, Ni G, Vasudevan S, Jagannath A, Foster RG (May 2025). "Dreaming of Better Treatments: Advances in Drug Development for Sleep Medicine and Chronotherapy". Journal of Sleep Research e70087. doi:10.1111/jsr.70087. PMID 40346938.
- ^ a b Chen E (2025-07-21). "Alkermes drug helped narcolepsy patients stay awake in Phase 2 trial". STAT. Retrieved 2025-07-30.