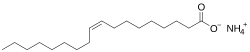
Ammonium oleate
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.008.067 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H37NO2 | |
| Molar mass | 299.499 g·mol−1 |
| Appearance | brown solid |
| Density | 0.903 g/cm3[1] |
| Melting point | 71 °C (decomposes)[2] |
| soluble | |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Ammonium oleate is a chemical compound with the chemical formula C17H33COONH4.[3][4][5] This is an organic ammonium salt of oleic acid.
Synthesis
Ammonium oleate is formed by the reaction between oleic acid and aqueous ammonia.[6]
Physical properties
Ammonium oleate emits toxic oxides of nitrogen when heated excessively. It is soluble in water.[7]
Uses
The compound is used in agriculture as a deer and rabbit repellent.[8][9]
References
- ^ "Ammonium oleate 100.008.067 | Density". European Chemicals Agency.
- ^ "CAS Common Chemistry | Ammonium oleate". Chemical Abstracts Service. Retrieved 8 March 2025.
- ^ Staff, ChemADVISOR Inc (6 December 2012). Regulated Chemicals Directory 1994. Springer Science & Business Media. p. 126. ISBN 978-94-011-0689-4. Retrieved 9 March 2025.
- ^ Technical Paper. U.S. Government Printing Office. 1917. p. 20. Retrieved 6 March 2025.
- ^ American Druggist and Pharmaceutical Record. American Druggist Publishing Company. 1895. p. 271. Retrieved 6 March 2025.
- ^ Allen, Alfred Henry (1899). Commercial Organic Analysis: pt. II. Hydrocarbons, petroleum and coal-tar products, asphalt, phenols and creosotes. 3d ed. with revisions and additions by the author and Henry Leffmann. 1900. J. & A. Churchill. p. 267. Retrieved 9 March 2025.
- ^ "AMMONIUM OLEATE | CAMEO Chemicals | NOAA". cameochemicals.noaa.gov. Retrieved 6 March 2025.
- ^ Dunn, Kevin M. (2010). Scientific Soapmaking: The Chemistry of the Cold Process. Clavicula Press. p. 182. ISBN 978-1-935652-09-0. Retrieved 6 March 2025.
- ^ Pesticides - Fact Sheet for Soap Salts (PDF). United States Environmental Protection Agency (Report). September 1992. EPA-738-F-92-013. Retrieved 8 March 2025.