Amyl nitrate
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Pentyl nitrate | |
| Other names
n-Amyl nitrate
1-Nitrooxypentane 1-Pentyl nitrate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.012.440 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 1112 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H11NO3 | |
| Molar mass | 133.147 g·mol−1 |
| Boiling point | 104 °C (219 °F; 377 K) |
| −76.4·10−6 cm3/mol | |
| Hazards | |
| GHS labelling: | |
 
| |
| Warning | |
| H226, H315, H319 | |
| P210, P233, P240, P241, P242, P243, P264, P280, P302+P352, P303+P361+P353, P305+P351+P338, P321, P332+P313, P337+P313, P362, P370+P378, P403+P235, P501 | |
| Flash point | 47.8 °C (118.0 °F; 320.9 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
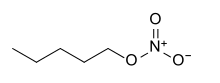
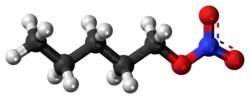
Amyl nitrate is the chemical compound with the formula CH3(CH2)4ONO2. This molecule consists of the 5-carbon amyl group attached to a nitrate functional group.[1] It is the ester of amyl alcohol and nitric acid.
Applications
Alkyl nitrates are employed as reagents in organic synthesis.[2] Amyl nitrate is used as an additive in diesel fuel, where it acts as an "ignition improver" (cetane improver) by accelerating the ignition of fuel.[3]
See also
- Amyl nitrite – a similarly named chemical used to treat heart diseases and cyanide poisoning
References
- ^ EPA on Pentyl nitrate
- ^ Zajac, W. W. Jr. (2001). "1-Nitropropane". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rn051. ISBN 0471936235.
- ^ "Amyl Nitrate | Cameo Chemicals | Noaa". Cameochemicals.noaa.gov. Retrieved 2016-09-22.