Bivamelagon
 | |
| Clinical data | |
|---|---|
| Other names | LB54640; LB-54640; LR-19021; LR19021 |
| Routes of administration | Oral |
| Drug class | Melanocortin MC4 receptor agonist |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
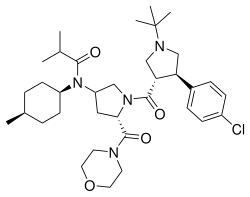
| Formula | C35H53ClN4O4 |
| Molar mass | 629.28 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Bivamelagon (INN; developmental code names LB54640, LR-19021) is a small-molecule melanocortin MC4 receptor agonist under development by LG Chem Life Sciences for the treatment of hypothalamic obesity, .[1][2] Unlike the older drug with the same mechanism of action, setmelanotide, it can be taken orally.[3][4][5] As of March 2024, it is in phase 2 clinical trials.[1]
References
- ^ a b "Rhythm Pharmaceuticals". AdisInsight. 13 March 2024. Retrieved 25 February 2025.
- ^ "Delving into the Latest Updates on Bivamelagon with Synapse". Synapse. 23 January 2025. Retrieved 25 February 2025.
- ^ Aronne, Sarah R. Barenbaum, Louis J. (2023). "Antiobesity Medications on the Horizon". Handbook of Obesity - Volume 2 (5 ed.). CRC Press. pp. 394–401. doi:10.1201/9781003432807-42. ISBN 978-1-003-43280-7.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ First-in-Human Study of Safety, Pharmacodynamics of LB54640, An Oral Melanocortin-4 Receptor Agonist Mirza, Victoria, MD, MPH; Lee, Jisoo, MD; Gwak, Heemin; Yang, Yunjeong; Kim, Mina. Obesity; Silver Spring Vol. 30, (Nov 2022): 145-146.
- ^ Piper, Noah B.C.; Whitfield, Emily A.; Stewart, Gregory D.; Xu, Xiaomeng; Furness, Sebastian G.B. (August 2022). "Targeting appetite and satiety in diabetes and obesity, via G protein-coupled receptors". Biochemical Pharmacology. 202: 115115. doi:10.1016/j.bcp.2022.115115. PMID 35671790. S2CID 249452717.