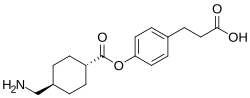
Cetraxate
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H23NO4 |
| Molar mass | 305.374 g·mol−1 |
| 3D model (JSmol) | |
| |
Cetraxate (INN) is an oral gastrointestinal medication which has a cytoprotective effect.[1][2]
Synthesis
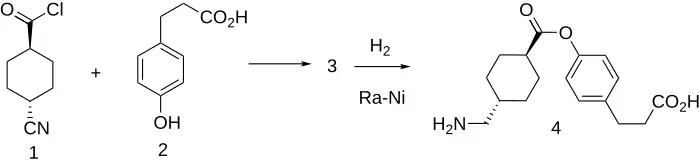
Cetraxate is a prodrug of tranexamic acid. The latter is a hemostatic agent because it inhibits the activation of plasminogen to plasmin. The result is to prevent excess loss of blood in gastrointestinal ulcers. The synthesis begins with the esterification of 3-(p-hydroxyphenyl)propionic acid (2) by trans-4-cyanocyclohexanecarbonyl chloride (1). The product (3) is reduced to (4) by catalytic hydrogenation with hydrogen and Raney nickel.[3][4]
References
- ^ Kurebayashi Y, Ikeda T, Osada Y (January 1988). "Cytoprotective action of cetraxate against HCl.ethanol-induced gastric lesion in rats". Japanese Journal of Pharmacology. 46 (1): 17–25. doi:10.1254/jjp.46.17. PMID 3367546.
- ^ Ishimori A, Yamagata S, Taima T (1979). "Effect of p-hydroxyphenyl-propionic ester of tranexamic acid hydrochloride (Cetraxate) on peptic ulcer. Multi-center clinical study". Arzneimittel-Forschung. 29 (10): 1625–1632. PMID 391240.
- ^ Japan Kokai, 59/134,758 (1984)
- ^ Svahn CM, Merenyi F, Karlson L, Widlund L, Grälls M (April 1986). "Tranexamic acid derivatives with enhanced absorption". Journal of Medicinal Chemistry. 29 (4): 448–453. doi:10.1021/jm00154a004. PMID 3959024.