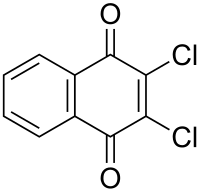
Dichlone
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,3-Dichloronaphthalene-1,4-dione | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.828 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 2902 2761 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H4Cl2O2 | |
| Molar mass | 227.04 g·mol−1 |
| Appearance | Yellow crystals[1] |
| Melting point | 193 °C (379 °F; 466 K)[1] |
| 0.1 ppm[1] | |
| Hazards | |
| GHS labelling:[1] | |
| |
| Warning | |
| H302, H315, H317, H319, H410 | |
| P261, P264, P264+P265, P270, P272, P273, P280, P301+P317, P302+P352, P305+P351+P338, P321, P330, P332+P317, P333+P317, P337+P317, P362+P364, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Dichlone (trade names Phygon and Quintar) is a fungicide and algicide of the quinone class. It is a general use fungicide applied to fruits, vegetables, field crops, ornamentals, and residential and commercial outdoor areas.[1] It is also used to control blue algae.[2]
Dichlone is not persistent in soil and has moderate mammalian toxicity.[2]
Dichlone can be manufactured by the chlorination and oxidation of naphthalene.[3]
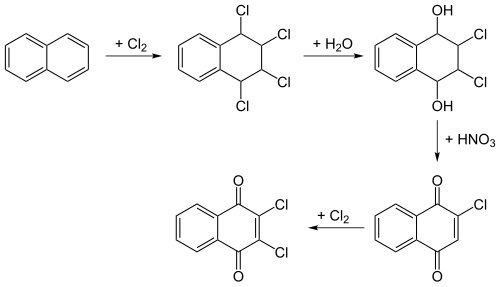
Synthesis of dichlone from naphthalene
References
- ^ a b c d "Dichlone (Phygon, Quintar) Chemical Profile". Pesticide Management Education Program, Cornell Cooperative Extension.
- ^ a b "Dichlone". Pesticide Properties DataBase, University of Hertfordshire.
- ^ Thomas A. Unger (1996). Pesticide Synthesis Handbook. William Andrew. p. 966. ISBN 0-8155-1853-6.