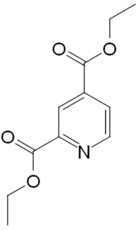
Diethyl lutidinate
 | |
| Clinical data | |
|---|---|
| Trade names | Stemoxydine; Mexoryl SBU |
| Other names | Diethyl 2,4-pyridinedicarboxylic acid 2,4-Pyridinedicarboxylic acid diethyl ester |
| Routes of administration | topical |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.114.112 |
| Chemical and physical data | |
| Formula | C11H13NO4 |
| Molar mass | 223.228 g·mol−1 |
| 3D model (JSmol) | |
| |
Diethyl lutidinate is a chemical compound. It has been studied for its potential use in hair care.[2]
It can be synthesized by reacting lutidinic acid with ethanol at elevated temperature in presence of sulfuric acid.[3]
References
- ^ a b c d "Diethyl lutidinate". pubchem. Archived from the original on February 16, 2018.
- ^ Jitsukawa S, Rathman-Josserand M, Bernard BA (2014). "Human hair follicle stem/progenitor cells and hypoxia: a new hair care approach with diethyl pyridine-2,4-dicarboxylate". Fragrance Journal (in Japanese). 42 (6): 12–19.
- ^ Shin, Hyunseo; Moon, Bongjin; Kwon, Hoejun. Transition metal complex containing tetradentate nitrogen donor ligand and electrochemical biosensor comprising same. 2022. WO 2022145982 A1.