Dysprosium arsenide
 | |
| Names | |
|---|---|
| Other names
Dysprosium monoarsenide, arsanylidynedysprosium
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| AsDy | |
| Molar mass | 237.422 g·mol−1 |
| Appearance | Crystalline |
| Density | g/cm3 |
| Related compounds | |
Other anions
|
Dysprosium nitride Dysprosium phosphide Dysprosium antimonide Dysprosium bismuthide |
Other cations
|
Terbium phosphide Holmium phosphide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Dysprosium arsenide is a binary inorganic compound of dysprosium and arsenide with the chemical formula DyAs.[1][2]
Physical properties
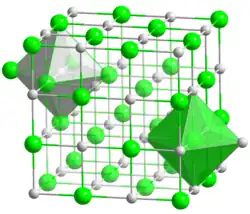
The compound is rock-salt structured, crystallizing in the cubic Fm3m space group.[3]
Uses
DyAs could be used as a semiconductor and in photo optic applications.[4][5]
Related
DyRuAsO is an arsenide oxide that also include ruthenium.[6]
References
- ^ "Dysprosium Arsenide". American Elements. Retrieved 28 May 2024.
- ^ Hwu, R. Jennifer; Wu, Ke (1999). Terahertz and Gigahertz Photonics: 19-23 July 1999, Denver, Colorado. SPIE. p. 217. ISBN 978-0-8194-3281-0. Retrieved 28 May 2024.
- ^ Standard X-ray Diffraction Powder Patterns. U.S. Government Printing Office. 1963. p. 53. Retrieved 28 May 2024.
- ^ "CAS 12005-81-1 Dysprosium Arsenide - Alfa Chemistry". alfa-chemistry.com. Retrieved 28 May 2024.
- ^ Ganjali, Mohammad Reza; Gupta, Vinod Kumar; Faridbod, Farnoush; Norouzi, Parviz (25 February 2016). Lanthanides Series Determination by Various Analytical Methods. Elsevier. p. 49. ISBN 978-0-12-420095-1. Retrieved 28 May 2024.
- ^ McGuire, Michael A.; May, Andrew F.; Sales, Brian C. (6 August 2012). "Crystallographic and Magnetic Phase Transitions in the Layered Ruthenium Oxyarsenides TbRuAsO and DyRuAsO". Inorganic Chemistry. 51 (15): 8502–8508. doi:10.1021/ic3010695. PMID 22835000.