Dicarboxylic acid
In organic chemistry, a dicarboxylic acid is an organic compound containing two carboxyl groups (−COOH). The general molecular formula for dicarboxylic acids can be written as HO2C−R−CO2H, where R can be aliphatic or aromatic.[1] In general, dicarboxylic acids show similar chemical behavior and reactivity to monocarboxylic acids.[1] Dicarboxylic acids are usually colorless solids. A wide variety of dicarboxylic acids are used in industry. Adipic acid, for example, is a precursor to certain kinds of nylon. A wide variety of dicarboxylic acids are found in nature. Aspartic acid and glutamic acid are two amino acids found in all life. Succinic and fumaric acids are essential for metabolism. A large inventory of derivatives are known including many mono- and diesters, amides, etc.[2]
Partial list of saturated dicarboxylic acids
Some common or illustrative examples
C n Common name Systematic IUPAC name Structure pKa1 pKa2 PubChem C2 0 Oxalic acid ethanedioic acid 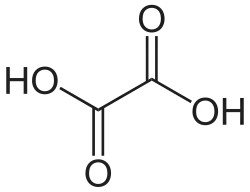
1.27 4.27 971 C3 1 Malonic acid propanedioic acid 
2.85 5.05 867 C4 2 Succinic acid butanedioic acid 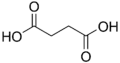
4.21 5.41 1110 C5 3 Glutaric acid pentanedioic acid 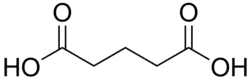
4.34 5.41 743 C6 4 Adipic acid hexanedioic acid 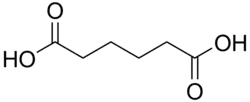
4.41 5.41 196 C7 5 Pimelic acid heptanedioic acid 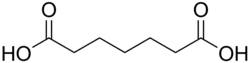
4.50 5.43 385 C8 6 Suberic acid octanedioic acid 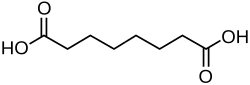
4.526 5.498 10457 C8 6 1,4-Cyclohexanedicarboxylic acid 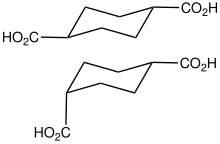
14106 C9 7 Azelaic acid nonanedioic acid 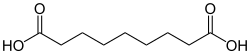
4.550 5.498 2266 C10 8 Sebacic acid decanedioic acid 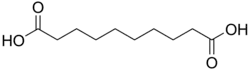
4.720 5.450 5192 C11 9 undecanedioic acid 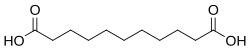
15816 C12 10 dodecanedioic acid 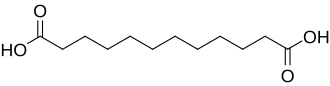
12736 C13 11 Brassylic acid tridecanedioic acid 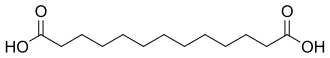
10458 C16 14 Thapsic acid hexadecanedioic acid 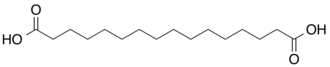
10459 C21 19 Japanic acid heneicosanedioic acid 9543668 C22 20 Phellogenic acid docosanedioic acid 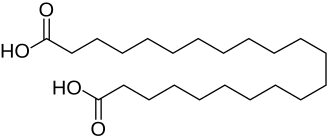
244872 C30 28 Equisetolic acid triacontanedioic acid 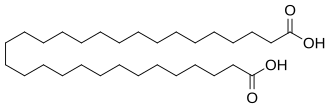
5322010
Unsaturated dicarboxylic acids
Type Common name IUPAC name Isomer Structural formula PubChem Monounsaturated Maleic acid (Z)-Butenedioic acid cis 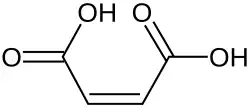
444266 Fumaric acid (E)-Butenedioic acid trans 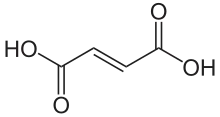
444972 Glutaconic acid (Z)-Pent-2-enedioic acid cis 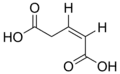
5370328 (E)-Pent-2-enedioic acid trans 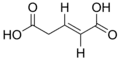
5280498 2-Decenedioic acid trans 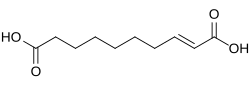
6442613 Traumatic acid Dodec-2-enedioic acid trans 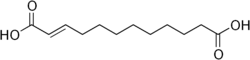
5283028 Diunsaturated Muconic acid (2E,4E)-Hexa-2,4-dienedioic acid trans,trans 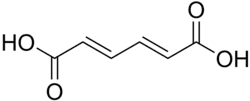
5356793 (2Z,4E)-Hexa-2,4-dienedioic acid cis,trans 
280518 (2Z,4Z)-Hexa-2,4-dienedioic acid cis,cis 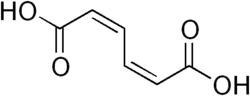
5280518 Glutinic acid
(Allene-1,3-dicarboxylic acid)(RS)-Penta-2,3-dienedioic acid HO2CCH=C=CHCO2H 5242834 Branched Citraconic acid (2Z)-2-Methylbut-2-enedioic acid cis 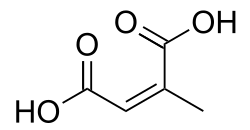
643798 Mesaconic acid (2E)-2-Methyl-2-butenedioic acid trans 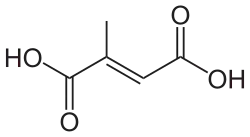
638129 Itaconic acid 2-Methylidenebutanedioic acid – 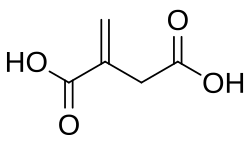
811 Acetylenic Acetylenedicarboxylic acid But-2-ynedioic acid not applicable 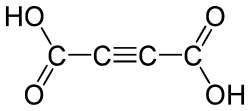
371
Substituted dicarboxylic acids
Common name IUPAC name Structural formula PubChem Tartronic acid 2-Hydroxypropanedioic acid 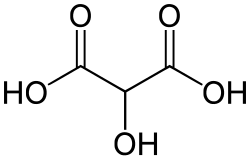
45 Mesoxalic acid Oxopropanedioic acid 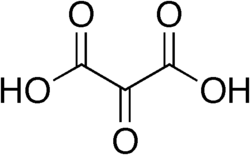
10132 Malic acid Hydroxybutanedioic acid 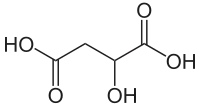
525 Tartaric acid 2,3-Dihydroxybutanedioic acid 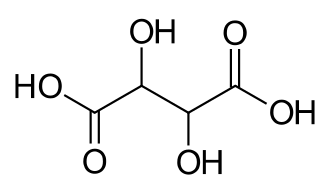
875 Oxaloacetic acid Oxobutanedioic acid 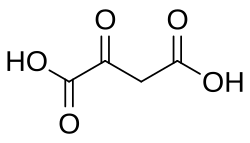
970 Aspartic acid 2-Aminobutanedioic acid 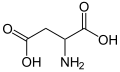
5960 dioxosuccinic acid dioxobutanedioic acid 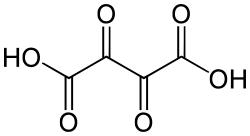
82062 α-hydroxyGlutaric acid 2-hydroxypentanedioic acid 
43 Arabinaric acid 2,3,4-Trihydroxypentanedioic acid 109475 Acetonedicarboxylic acid 3-Oxopentanedioic acid 
68328 α-Ketoglutaric acid 2-Oxopentanedioic acid 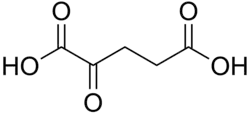
51 Glutamic acid 2-Aminopentanedioic acid 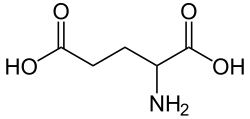
611 Diaminopimelic acid (2R,6S)-2,6-Diaminoheptanedioic acid 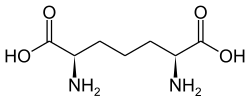
865 Saccharic acid (2S,3S,4S,5R)-2,3,4,5-Tetrahydroxyhexanedioic acid 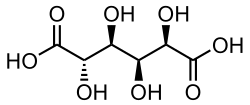
33037
Aromatic dicarboxylic acids
Common names IUPAC name Structure PubChem Phthalic acid
o-phthalic acidBenzene-1,2-dicarboxylic acid 
1017 Isophthalic acid
m-phthalic acidBenzene-1,3-dicarboxylic acid 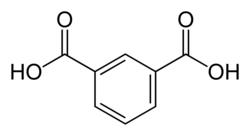
8496 Terephthalic acid
p-phthalic acidBenzene-1,4-dicarboxylic acid 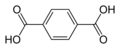
7489 Diphenic acid
Biphenyl-2,2′-dicarboxylic acid2-(2-Carboxyphenyl)benzoic acid 
10210 2,6-Naphthalenedicarboxylic acid 2,6-Naphthalenedicarboxylic acid 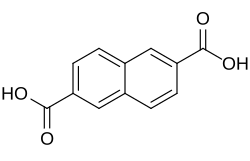
14357
Terephthalic acid is a commodity chemical used in the manufacture of the polyester known by brand names such as PET, Terylene, Dacron and Lavsan.
See also
References
- ^ a b Boy Cornils, Peter Lappe "Dicarboxylic Acids, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry 2014, Wiley-VCH, Weinheim. doi:10.1002/14356007.a08_523.pub3
- ^ Bernthsen, A. (1922). Organic Chemistry. London: Blackie & Son. p. 242.
External links
- Lipidomics gateway Structure Database Dicarboxylic acids
- Dijkstra, Albert J. "Trivial names of fatty acids-Part 1". lipidlibrary.aocs.org. Retrieved 24 June 2019.