Feclemine
 | |
| Clinical data | |
|---|---|
| Other names |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
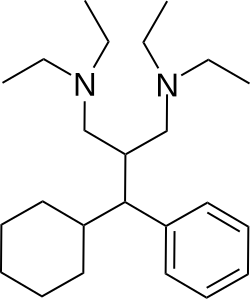
| Formula | C24H42N2 |
| Molar mass | 358.614 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Feclemine (phenetamine) is a spasmolytic drug.[1]
Synthesis
The chemical synthesis is discussed:[2]
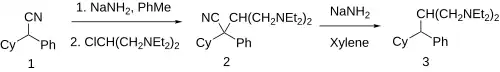
Sodamide is used to alkylate cyclohexylphenylacetonitrile [3893-23-0] (1) (also used for drofenine) with 2-chloro-N,N,N',N'-tetraethylpropane-1,3-diamine [3492-54-4] (HCl: [94465-65-3]) to give (2). Some rearrangement is possible to give a mixture of regioisomers. Sodamide catalyzed cleavage of the nitrile group in a separate step then completes the synthesis of feclemine (3).

References
- ^ Crema A, Benzi G, Frigo GM, Berté F (July 1965). "Method for evaluating spasmolytic activity of drugs on the bile duct". The Journal of Pharmacy and Pharmacology. 17 (7): 405–408. doi:10.1111/j.2042-7158.1965.tb07695.x. PMID 4379262.
- ^ Morren HG, Denayer R, Trolin S, Strubbe H, Linz R, Dony G, et al. (1955). "Antispasmodics with musculotropic action derived from 1,3-bis(dialkylamino)propane". Industrie Chimique Belge. 20: 733–745.
- ^ a b Schütz S, Kurz J, Plümpe H, Bock M, Otten H (June 1971). "[Basically alkylated imides of naphthalene-1,4,5,8-tetracarboxylic acid and their chemotherapeutic effects]". Arzneimittel-Forschung (in German). 21 (6): 739–763. PMID 4998088.