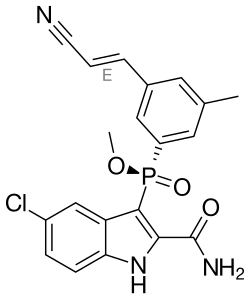
Fosdevirine
 | |
| Clinical data | |
|---|---|
| Other names | GSK2248761; GSK2248761A; IDX-12899; IDX-899 |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C20H17ClN3O3P |
| Molar mass | 413.80 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Fosdevirine is an experimental antiviral agent of the non-nucleoside reverse transcriptase inhibitor class that was studied for potential use in the treatment of HIV-AIDS.[1][2]
It was discovered by Idenix Pharmaceuticals and was being developed by GlaxoSmithKline and ViiV Healthcare, but it has now been discontinued[3][4] due to unexpected side effects.[5]
References
- ^ Dousson C, Alexandre FR, Amador A, Bonaric S, Bot S, Caillet C, et al. (March 2016). "Discovery of the Aryl-phospho-indole IDX899, a Highly Potent Anti-HIV Non-nucleoside Reverse Transcriptase Inhibitor". Journal of Medicinal Chemistry. 59 (5): 1891–8. doi:10.1021/acs.jmedchem.5b01430. PMID 26804933.
- ^ "Fosdevirine". AIDSinfo. U.S. National Library of Medicine, U.S. Department of Health and Human Services. Archived from the original on 2019-08-11. Retrieved 2018-08-14.
- ^ "Fosdevirine". Adis Insight. Springer Nature Switzerland AG.
Highest Development Phases: Discontinued, HIV-1 infections
- ^ "Fosdevirine". MedKoo Biosciences.
GSK2248761 is no longer in clinical development.
- ^ Margolis DA, Eron JJ, DeJesus E, White S, Wannamaker P, Stancil B, Johnson M (2013). "Unexpected finding of delayed-onset seizures in HIV-positive, treatment-experienced subjects in the Phase IIb evaluation of fosdevirine (GSK2248761)". Antiviral Therapy. 19 (1): 69–78. doi:10.3851/IMP2689. PMID 24158593. S2CID 6147197.