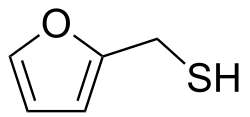
Furan-2-ylmethanethiol
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(Furan-2-yl)methanethiol | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 383594 | |
| ChemSpider | |
| ECHA InfoCard | 100.002.390 |
| EC Number |
|
| MeSH | furfuryl+mercaptan |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3336 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H6OS | |
| Molar mass | 114.16 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | Roasted coffee, caramel, sulfurous, waxy |
| Density | 1.132 g cm−3 |
| Boiling point | 155 °C; 311 °F; 428 K |
| Vapor pressure | 531 Pa |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H226 | |
| Flash point | 45 °C (113 °F; 318 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
100-200 mg kg−1 (mouse) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Furan-2-ylmethanethiol (2-furanmethanethiol) is an organosulfur compound. It is classified as a furan substituted with a methylthiol group. It is a colourless liquid, but impure samples can appear yellow. It possesses a strong odour of roasted coffee[1] and a bitter taste. It is a key component of the aroma of roasted coffee. It has been identified as a trigger molecule for parosmia following COVID-19 infection.[2][3]
Synthesis
Furan-2-ylmethanethiol is prepared by treating furfuryl alcohol with thiourea in hydrochloric acid via an intermediate isothiouronium salt, which is hydrolized to the thiol by heating with sodium hydroxide.[4]

Synthesis of furfuryl mercaptane (Furan-2-ylmethanethiol)
References
- ^ Viani, Rinantonio; Petracco, Marino (2007). "Coffee". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a07_315.pub2. ISBN 978-3-527-30385-4.
- ^ Parker JK, Kelly CE, Gane SB (5 February 2021). "Molecular Mechanism of Parosmia". p. 21251085. medRxiv 10.1101/2021.02.05.21251085.
- ^ Devlin H (25 May 2022). "Scientists identify 'trigger molecule' for Covid-related changes to smell". The Guardian.
- ^ "Preparation of Furfuryl mercaptan". Organic Syntheses. 35: 66. 1955. doi:10.15227/orgsyn.035.0066.