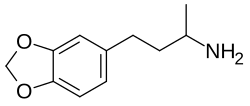
Homo-MDA
 | |
| Clinical data | |
|---|---|
| Other names | HMDA α-Methyl-γ-(3,4-methylenedioxyphenyl)propylamine; α-Methyl-1,3-benzodioxole-5-propanamine; 3,4-Methylenedioxyphenylaminobutane |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.359.348 |
| Chemical and physical data | |
| Formula | C11H15NO2 |
| Molar mass | 193.246 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Homo-MDA (HMDA), also known as α-methyl-γ-(3,4-methylenedioxyphenyl)propylamine, is a entactogen-like drug related to 3,4-methylenedioxyamphetamine (MDA).[1] It is an analogue of MDA in which the side chain has been lengthened by one carbon atom.[1]
Unlike the stimulant amphetamine and the psychedelic DOM, and in contrast to MDA, homo-MDA has no effect on locomotor activity in rodents.[2] It was found to be more toxic than MDA in rodents.[3]
The effects of homo-MDA in humans are unknown.[1] It is not a controlled substance in the United States as of 2011.[1]
See also
References
- ^ a b c d Shulgin A, Manning T, Daley PF (2011). "#78. Homo-MDA". The Shulgin Index, Volume One: Psychedelic Phenethylamines and Related Compounds. Vol. 1. Berkeley, CA: Transform Press. pp. 177–179. ISBN 978-0-9630096-3-0. OCLC 709667010.
- ^ Buxton DA (1972). "Behavioural Actions of Some Substituted Amphetamines". Biochemical and Pharmacological Mechanisms Underlying Behaviour. Progress in Brain Research. Vol. 36. pp. 171–181. doi:10.1016/S0079-6123(08)62519-4. ISBN 978-0-444-40992-8. PMID 4644027.
- ^ Davis WM, Borne RF (1984). "Pharmacologic investigation of compounds related to 3,4-methylenedioxyamphetamine (MDA)". Substance and Alcohol Actions/Misuse. 5 (2): 105–110. PMID 6147902.