Lithium hexafluoroantimonate
 | |
| Names | |
|---|---|
| IUPAC name
lithium;hexafluoroantimony(1-)
| |
| Other names
Lithium hexafluoroantimonate(V)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.196.093 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| NaSbF6 | |
| Appearance | White to off-white powder |
| soluble | |
| Hazards | |
| GHS labelling: | |
| |
| Warning | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Lithium hexafluoroantimonate is an inorganic chemical compound with the chemical formula LiSbF6.[1][2][3]
Physical properties
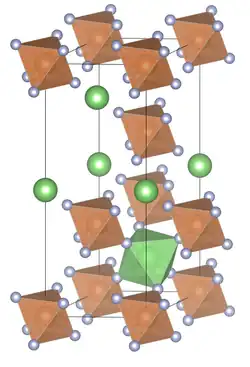
The compound crystallizes in the rhombohedral space group R3 with the lattice constants a = 5.43 Å, α = 56° 58', Z = 1. The structure corresponds to that of rhombohedral distorted NaSbF6 type.[4][5]
References
- ^ "Lithium hexafluoroantimonate(V)". Sigma Aldrich. Retrieved 1 July 2024.
- ^ Haynes, William M. (9 June 2015). CRC Handbook of Chemistry and Physics, 96th Edition. CRC Press. p. 4-72. ISBN 978-1-4822-6097-7. Retrieved 1 July 2024.
- ^ Manthiram, Arumugam (March 2009). Rechargeable Lithium and Lithium Ion Batteries. The Electrochemical Society. p. 157. ISBN 978-1-56677-704-9. Retrieved 1 July 2024.
- ^ Burns, J. H. (10 November 1962). "The crystal structure of lithium fluoroantimonate(V)". Acta Crystallographica. 15 (11): 1098–1101. doi:10.1107/S0365110X62002935. ISSN 0365-110X. Retrieved 1 July 2024.
- ^ Donnay, Joseph Désiré Hubert (1978). Crystal Data: Inorganic compounds 1967-1969. National Bureau of Standards. p. H/R-188. Retrieved 1 July 2024.