Lutetium(III) chloride
 | |
| Names | |
|---|---|
| IUPAC name
Lutetium(III) chloride
| |
| Other names
Lutetium chloride, lutetium trichloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.205 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| LuCl3 | |
| Molar mass | 281.325 g/mol |
| Appearance | colorless or white monoclinic crystals |
| Density | 3.98 g/cm3 |
| Melting point | 925 °C (1,697 °F; 1,198 K)[3] |
| Boiling point | sublimes above 750°C[1] |
| soluble[2] | |
| Structure | |
| Monoclinic, mS16 | |
| C2/m, No. 12 | |
| Pharmacology | |
| License data | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant |
| GHS labelling:[4][5] | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
Lutetium(III) oxide |
Other cations
|
Ytterbium(III) chloride Scandium(III) chloride Yttrium(III) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
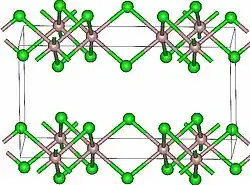
Lutetium(III) chloride or lutetium trichloride is the chemical compound composed of lutetium and chlorine with the formula LuCl3. It forms hygroscopic white monoclinic crystals[3] and also a hygroscopic hexahydrate LuCl3·6H2O.[6] Anhydrous lutetium(III) chloride has the YCl3 (AlCl3) layer structure with octahedral lutetium ions.[7]
Lutetium-177, a radioisotope that can be derived from lutetium(III) chloride, is used in targeted cancer therapies.[8] When lutetium-177 is attached to molecules that specifically target cancer cells, it can deliver localized radiation to destroy those cells while sparing surrounding healthy tissue.[9] This makes lutetium-177-based treatments especially valuable for cancers that are difficult to treat with traditional methods, such as neuroendocrine tumors and prostate cancer.[10] Additionally, lutetium(III) chloride is used in scintillators, materials that emit light when exposed to radiation.[11] These scintillators are crucial in detectors for gamma rays and other high-energy particles, used in both medical diagnostics and in scientific research.[12]
Reactions
Pure lutetium metal can be produced from lutetium(III) chloride by heating it together with elemental calcium:[13]
See also
References
- ^ "Chemistry: Periodic Table: Lutetium: compound data (lutetium (III) chloride)". WebElements. Retrieved 2024-09-06.
- ^ Perry, Dale L.; Phillips, Sidney L. (1995), Handbook of Inorganic Compounds, CRC Press, p. 232, ISBN 0-8493-8671-3, retrieved 2008-06-27
- ^ a b Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, p. 472, ISBN 0-8493-0594-2, retrieved 2008-06-27
- ^ "450960 Lutetium(III) chloride anhydrous, powder, 99.99% trace metals basis". Sigma-Aldrich. Retrieved 2008-06-27.
- ^ "Lutetium chloride". pubchem.ncbi.nlm.nih.gov.
- ^ "Lutetium(III) chloride hexahydrate 542075". Sigma-Aldrich. Retrieved 2019-07-24.
- ^ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- ^ Sgouros, George; Bodei, Lisa; McDevitt, Michael R.; Nedrow, Jessie R. (September 2020). "Radiopharmaceutical therapy in cancer: clinical advances and challenges". Nature Reviews Drug Discovery. 19 (9): 589–608. doi:10.1038/s41573-020-0073-9. ISSN 1474-1784. PMC 7390460.
- ^ Vyas, Madhusudan (2021-05-01). "Lutetium-177: a flexible radionuclide therapeutic options". Journal of Nuclear Medicine. 62 (supplement 1): 3039. ISSN 0161-5505.
- ^ Dash, Ashutosh; Pillai, Maroor Raghavan Ambikalmajan; Knapp, Furn F. (2015-06-01). "Production of 177Lu for Targeted Radionuclide Therapy: Available Options". Nuclear Medicine and Molecular Imaging. 49 (2): 85–107. doi:10.1007/s13139-014-0315-z. ISSN 1869-3482. PMC 4463871. PMID 26085854.
- ^ Vogel, W. V.; van der Marck, S. C.; Versleijen, M. W. J. (2021-07-01). "Challenges and future options for the production of lutetium-177". European Journal of Nuclear Medicine and Molecular Imaging. 48 (8): 2329–2335. doi:10.1007/s00259-021-05392-2. ISSN 1619-7089. PMC 8241800. PMID 33974091.
- ^ Das, Tapas; Banerjee, Sharmila (2016). "Theranostic Applications of Lutetium-177 in Radionuclide Therapy". Current Radiopharmaceuticals. 9 (1): 94–101. doi:10.2174/1874471008666150313114644. ISSN 1874-4729. PMID 25771364.
- ^ Patnaik, Pradyot (2004), Handbook of Inorganic Chemicals, Amsterdam: McGraw-Hill Professional, p. 244, ISBN 0-07-049439-8, retrieved 2008-06-27
