MRTX1133
 | |
| Clinical data | |
|---|---|
| Drug class | Antineoplastic agents |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
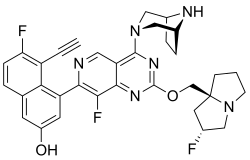
| Formula | C33H31F3N6O2 |
| Molar mass | 600.646 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
MRTX1133 is an investigational drug that targets the G12D mutation in KRAS dependent cancers.[1][2] MRTX1133 was in a phase 1/2 clinical trial for the treatment of solid tumors,[3] however the study was terminated in Q1 2025.[4] MRTX1133 is considered to be harmful from direct skin or eye exposure other than transient irritation. It may cause irritation of the respiratory system if inhaled.[5]
See also
References
- ^ "MRTX 1133". AdisInsight. Springer Nature Switzerland AG.
- ^ Zeissig MN, Ashwood LM, Kondrashova O, Sutherland KD (November 2023). "Next batter up! Targeting cancers with KRAS-G12D mutations". Trends in Cancer. 9 (11): 955–967. doi:10.1016/j.trecan.2023.07.010. PMID 37591766.
- ^ Clinical trial number NCT05737706 for "Study of MRTX1133 in Patients With Advanced Solid Tumors Harboring a KRAS G12D Mutation" at ClinicalTrials.gov
- ^ "Bristol exits KRAS G12D". ApexOnco. Retrieved 22 June 2025.
- ^ "MRTX1133 Safety Data Sheet". TargetMol. Retrieved 11 September 2024.