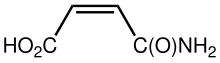
Maleamic acid
 | |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.332 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H5NO3 | |
| Molar mass | 115.088 g·mol−1 |
| Appearance | white solid |
| Melting point | 158–161 °C (316–322 °F; 431–434 K) |
| Hazards | |
| GHS labelling:[1] | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Maleamic acid is an organic compound with the formula HO2CCH=CHC(O)NH2. It is the amide formed by reaction of maleic anhydride with ammonia. It is a colorless solid.[1] Maleamic acid is the product of the action of the enzyme maleimide hydrolase.
Related compounds
A variety of maleamic acids are known. Commonly they are prepared by the reaction of an amine with maleic anhydride.[2]
Aside from maleic anhydride, other common cyclic anhydrides (and imides) undergo ring-opening to give amido carboxylic acids. Succinic anhydride gives succinamic acid (HO2CCH2CH2C(O)NH2), citraconic anhydride gives two isomers of citraconamic acid (HO2CCH2CH(CH3)C(O)NH2 and HO2CCH(CH3)CH2C(O)NH2), and phthalimide gives phthalamidic acid (HO2CC6H4C(O)NH2).[3]
References
- ^ Hood, David K. (2024). "Maleic Anhydride, Maleic Acid, and Fumaric Acid". Kirk-Othmer Encyclopedia of Chemical Technology. pp. 1–51. doi:10.1002/0471238961.1301120506051220.a01.pub3. ISBN 978-0-471-48494-3.
- ^ Mehta, Nariman B.; Phillips, Arthur P.; Lui, (MRS.) Florence FU; Brooks, Ronald E. (1960). "Maleamic and Citraconamic Acids, Methyl Esters, and Imides". The Journal of Organic Chemistry. 25 (6): 1012–1015. doi:10.1021/jo01076a038.
- ^ Hargreaves, Michael K.; Pritchard, J. G.; Dave, H. R. (1970). "Cyclic Carboxylic Monoimides". Chemical Reviews. 70 (4): 439–469. doi:10.1021/cr60266a001.