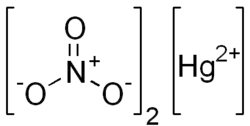
Mercury(II) nitrate
 | |
 | |
| Names | |
|---|---|
| IUPAC names
Mercury dinitrate
Mercury(II) nitrate | |
| Other names
Mercuric nitrate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.126 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1625 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Hg(NO3)2 | |
| Molar mass | 324.60 g/mol (anhydrous) |
| Appearance | colorless crystals or white powder |
| Odor | sharp |
| Density | 4.3 g/cm3 (monohydrate) |
| Melting point | 79 °C (174 °F; 352 K) (monohydrate) |
| soluble | |
| Solubility | soluble in nitric acid, acetone, ammonia insoluble in ethanol |
| −74.0·10−6 cm3/mol | |
| Hazards | |
| GHS labelling: | |
| |
| Danger | |
| H272, H300, H310, H330, H373, H410 | |
| NFPA 704 (fire diamond) | |
| Flash point | Nonflammable |
| Safety data sheet (SDS) | ICSC 0980 |
| Related compounds | |
Other anions
|
Mercury(II) sulfate Mercury(II) chloride |
Other cations
|
Zinc nitrate Cadmium nitrate |
Related compounds
|
Mercury(I) nitrate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Mercury(II) nitrate is an inorganic compound with the chemical formula Hg(NO3)2. It is the mercury(II) salt of nitric acid HNO3. It contains mercury(II) cations Hg2+ and nitrate anions NO−3, and water of crystallization H2O in the case of a hydrous salt. Mercury(II) nitrate forms hydrates Hg(NO3)2·xH2O. Anhydrous and hydrous salts are colorless or white soluble crystalline solids that are occasionally used as a reagents. Mercury(II) nitrate is made by treating mercury with hot concentrated nitric acid. Neither anhydrous nor monohydrate has been confirmed by X-ray crystallography.[1] The anhydrous material is more widely used.
Uses
Mercury(II) nitrate is used as an oxidizing agent in organic synthesis, as a nitrification agent, as an analytical reagent in laboratories, in the manufacture of felt, and in the manufacture of mercury fulminate.[2] An alternative qualitative Zeisel test can be done with the use of mercury(II) nitrate instead of silver nitrate, leading to the formation of scarlet red mercury(II) iodide.[3]
Health information
Mercury compounds are highly toxic. The use of this compound by hatters and the subsequent mercury poisoning of said hatters is a common theory of where the phrase "mad as a hatter" came from.
See also
References
- ^ Nolte, M.; Pantenburg, I.; Meyer, G. (9 December 2005). "The Monohydrate of Basic Mercuric Nitrate, [Hg(OH)](NO3)(H2O)". Zeitschrift für anorganische und allgemeine Chemie (in German). 632 (1). Wiley Publishing: 111–113. doi:10.1002/zaac.200500344. ISSN 0044-2313. Archived from the original on 27 November 2021. Retrieved 16 May 2022.
- ^ "Mercury nitrate monohydrate". Chemical Book. 2023. Retrieved June 30, 2024.
- ^ Wang, Zerong (2010). "Zeisel Determination". Comprehensive Organic Name Reactions and Reagents. John Wiley & Sons. pp. 3115–3118. doi:10.1002/9780470638859.conrr689. ISBN 9780470638859.
External links
- ATSDR - Toxic Substances Portal - Mercury (11/14/2013)
- ATSDR - Public Health Statement: Mercury (11/14/2013)
- ATSDR - ALERT! Patterns of Metallic Mercury Exposure, 6/26/97 (link not traceable 11/14/2013)
- ATSDR - Medical Management Guidelines for Mercury (11/14/2013)
- ATSDR - Toxicological Profile: Mercury (11/14/2013)
- Safety data (MSDS) (link not traceable 11/14/2013)
- Mercuric Nitrate (ICSC)
- Mercury Archived 2018-02-17 at the Wayback Machine
- Mercury Information Packages
- How to Make Good Mercury Electrical Connections, Popular Science monthly, February 1919, Unnumbered page, Scanned by Google Books: https://books.google.com/books?id=7igDAAAAMBAJ&pg=PT14
