Methyl violet 2B
 | |
| Names | |
|---|---|
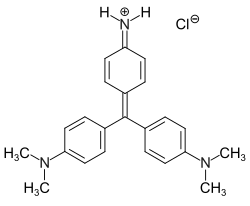
| IUPAC name
4,4′-[(4-Imino-2,5-cyclohexadien-1-yliden)methylen]bis(N,N-dimethylaniline)hydrochloride
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.074.935 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C23H26N3Cl | |
| Appearance | Green to dark-green powder[1] |
| Melting point | decomposes[1] |
| Soluble in water, ethanol, insoluble in xylene[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Methyl violet 2B (Tetramethylparosanilinium chloride, 4,4′-[(4-imino-2,5-cyclohexadien-1-yliden)methylen]bis(N,N-dimethylaniline)hydrochloride) is a violet triarylmethane dye from the group of cationic dyes and an essential component of C.I. Basic Violet 1 (trivial name methyl violet). Methyl violets are mixtures of tetramethyl (2B), pentamethyl (6B) and hexamethyl (10B) pararosanilins.[2]
References
- ^ a b c R. W. Sabnis (29 March 2010). Handbook of Biological Dyes and Stains: Synthesis and Industrial Applications. John Wiley and Sons. pp. 309–. ISBN 978-0-470-40753-0. Retrieved 27 June 2011.
- ^ Bouasla, C.; Samar, M. E. H.; Ismail, F. (2010). "Degradation of methyl violet 6B dye by the Fenton process". Desalination. 254 (1–3): 35–41. doi:10.1016/j.desal.2009.12.017.