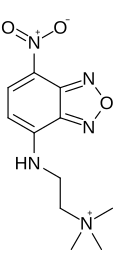
NBD-TMA
 | |
| Names | |
|---|---|
| Preferred IUPAC name
N,N,N-Trimethyl-2-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]ethan-1-aminium | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H16N5O3+ | |
| Molar mass | 266.280 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
NBD-TMA (2-(4-nitro-2,1,3-benzoxadiazol-7-yl)aminoethyl]trimethylammonium) is a small, positively charged (+1) fluorescent dye. It was also known as EAM-1 (N,N,N,-trimethyl-2[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]ethanaminium iodide) when it was briefly supplied by Macrocyclics Company as an iodide complex.
NBD-TMA has an excitation maximum at 458 nm and an emission maximum at 530 nm. It also has a smaller local excitation maximum around 343 nm. The molar extinction coefficient is about 13,000 cm−1M−1 and its overall effective fluorescence is about 1% that of fluorescein. It is only mildly sensitive to halide ion collision quenching.
NBD-TMA was designed as a probe for monitoring renal transport of organic cations.[1] As a small, positively charged fluorophore, it has also seen use as a tracer for measuring gap junction coupling in cases of cation selective connexin channels.[2]
References
- ^ Bednarczyk, D.; Mash, E. A.; Aavula, B. R.; Wright, S. H. (2000). "NBD-TMA: A novel fluorescent substrate of the peritubular organic cation transporter of renal proximal tubules". Pflügers Archiv: European Journal of Physiology. 440 (1): 184–192. doi:10.1007/s004240000283. PMID 10864014. S2CID 2699877.
- ^ Zhao, H. B. (2005). "Connexin26 is responsible for anionic molecule permeability in the cochlea for intercellular signalling and metabolic communications". The European Journal of Neuroscience. 21 (7): 1859–1868. doi:10.1111/j.1460-9568.2005.04031.x. PMC 2548270. PMID 15869481.