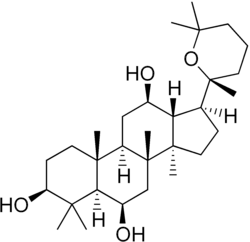
Panaxatriol
 | |
| Names | |
|---|---|
| IUPAC name
(20R)-20,25-Epoxydammarane-3β,6β,12β-triol
| |
| Systematic IUPAC name
(1S,3aR,3bR,5R,5aR,7S,9aR,9bR,11R,11aR)-3a,3b,6,6,9a-Pentamethyl-1-[(2R)-2,6,6-trimethyloxan-2-yl]hexadecahydro-1H-cyclopenta[a]phenanthrene-5,7,11-triol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.208.677 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H52O4 | |
| Molar mass | 476.742 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Panaxatriol is an organic compound that is an aglycone of ginsenosides, a group of steroid glycosides.[1] It is a dammarane-type tetracyclic triterpene sapogenin found in ginseng (Panax ginseng) and in notoginseng (Panax pseudoginseng). It is formed by the dehydration of protopanaxatriol.
See also
References
- ^ Kang, Soo Yeon; Schini-Kerth, Valérie B.; Kim, Nak Doo (1995). "Ginsenosides of the protopanaxatriol group cause endothelium-dependent relaxation in the rat aorta". Life Sciences. 56 (19): 1577–1586. doi:10.1016/0024-3205(95)00124-o. PMID 7723586.