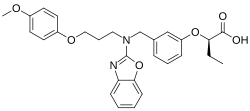
Pemafibrate
 | |
| Clinical data | |
|---|---|
| Trade names | Parmodia |
| Other names | K-13675 |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C28H30N2O6 |
| Molar mass | 490.556 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Pemafibrate, sold under the brand name Parmodia, is a peroxisome proliferator-activated receptor alpha (PPARα) agonist. It is developed and marketed by Kowa Pharmaceuticals.[1]
In July 2017, Pharmaceuticals and Medical Devices Agency approved it in Japan.[2]
References
- ^ Yamashita S, Masuda D, Matsuzawa Y (January 2020). "Pemafibrate, a New Selective PPARα Modulator: Drug Concept and Its Clinical Applications for Dyslipidemia and Metabolic Diseases". Current Atherosclerosis Reports. 22 (1): 5. doi:10.1007/s11883-020-0823-5. PMC 6978439. PMID 31974794.
- ^ Pemafibrate Archived 2018-02-05 at the Wayback Machine, pharmacodia.com