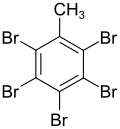
Pentabromotoluene
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,2,3,4,5-pentabromo-6-methylbenzene | |
| Other names
Pentabromomethylbenzene, 2,3,4,5,6-Pentabromotoluene, PBT
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.001.614 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H3Br5 | |
| Molar mass | 486.621 g·mol−1 |
| Appearance | white crystals |
| Density | 1.67 g/cm³ |
| Melting point | 290 °C (554 °F; 563 K) |
| soluble | |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Pentachlorotoluene is a synthetic organobromine compound with the molecular formula C6Br5CH3.[1][2]
Synthesis
Pentabromotoluene is a derivative of toluene and is synthesized from it.[3]
Physical properties
The compound forms white crystalline powder. Its crystals are of monoclinic system.[4] Due to the substitution with five bromine atoms on the aromatic ring, pentabromotoluene has a significantly lower volatility than toluene.
Uses
Pentabromotoluene is widely used as a flame retardant in textiles, rubber, unsaturated polyesters, polyethylene, SBR latex, etc.[5][6]
See also
References
- ^ "2,3,4,5,6-Pentabromotoluene 98.0+%, TCI America - Chemicals, Organic compounds". Fisher Scientific. Retrieved 22 April 2025.
- ^ "Pentabromotoluene". drugs.ncats.io. Retrieved 22 April 2025.
- ^ Nevile, R. H. C.; Winther, A. (1880). "Die sechs Tribromtoluole, die drei Tetrabromtoluole und das Pentabromtoluol". Berichte der deutschen chemischen Gesellschaft. 13 (1): 974–976. doi:10.1002/cber.188001301273. ISSN 1099-0682. Retrieved 22 April 2025.
- ^ Krigbaum, W. R.; Wildman, G. C. (15 December 1971). "The crystal structure of pentabromotoluene". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry. 27 (12): 2353–2358. Bibcode:1971AcCrB..27.2353K. doi:10.1107/S0567740871005879. ISSN 0567-7408. Retrieved 22 April 2025.
- ^ "2,3,4,5,6-Pentabromotoluene". Sigma Aldrich. Retrieved 22 April 2025.
- ^ Milne, G. W. A. (19 August 2005). Gardner's Commercially Important Chemicals: Synonyms, Trade Names, and Properties. John Wiley & Sons. p. 474. ISBN 978-0-471-73661-5. Retrieved 22 April 2025.