Pipradimadol
 | |
| Clinical data | |
|---|---|
| Other names | FU 29-245 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
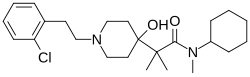
| Formula | C24H37ClN2O2 |
| Molar mass | 421.02 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Pipradimadol (FU 29-245) is an analgesic, antiserotoninergic agent and potentially an antidepressant.[1][2] The pharmacology is said to be mediated through the opiate receptor. The potency is on the same order of magnitude as for morphine.[3]
See also
References
- ^ Huegi BS, Ebnöther AM, Rissi E, Gadient F, Hauser D, Roemer D, et al. (January 1983). "Synthesis and pharmacological studies of 4,4-disubstituted piperidines: a new class of compounds with potent analgesic properties". Journal of Medicinal Chemistry. 26 (1): 42–50. doi:10.1021/jm00355a010. PMID 6600791.
- ^ Oswald I, Adam K, Spiegel R (November 1982). "Human EEG slow-wave sleep increased by a serotonin antagonist". Electroencephalography and Clinical Neurophysiology. 54 (5): 583–6. doi:10.1016/0013-4694(82)90044-x. PMID 6181982.
- ^ US 4178377, Rissi E, Ebnother A, "(4-Hydroxy-4-piperidyl)-alkanoic acid amides", issued 11 December 1979, assigned to Sandoz AG.
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
This article is issued from Wikipedia. The text is available under Creative Commons Attribution-Share Alike 4.0 unless otherwise noted. Additional terms may apply for the media files.