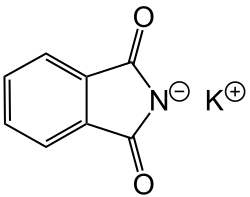
Potassium phthalimide
 | |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.012.770 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H4KNO2 | |
| Molar mass | 185.221 g/mol |
| Appearance | Light yellow solid |
| Melting point | > 300 °C (572 °F; 573 K) |
| Soluble in water | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related compounds
|
Phthalimide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Potassium phthalimide is a chemical compound of formula C8H4KNO2. It is the potassium salt of phthalimide, and usually presents as fluffy, very pale yellow crystals. It can be prepared by adding a hot solution of phthalimide in ethanol to a solution of potassium hydroxide in ethanol; the desired product precipitates.[1]
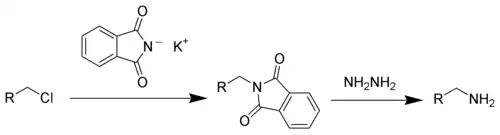
This compound is a commercially available reagent used in the Gabriel synthesis of amines.
References
- ^ P. L. Salzberg and J. V. Supniewski (1941). "β-Bromoethylphthalimide". Organic Syntheses; Collected Volumes, vol. 1, p. 119.
