Prokaryotic phospholipase A2
| Prokaryotic phospholipase A2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
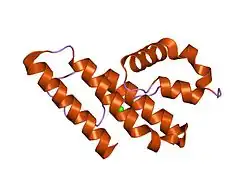 Structure of prokaryotic phospholipase A2.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | Phospholip_A2_3 | ||||||||
| Pfam | PF09056 | ||||||||
| InterPro | IPR015141 | ||||||||
| OPM superfamily | 82 | ||||||||
| OPM protein | 1kp4 | ||||||||
| |||||||||
The prokaryotic phospholipase A2 domain is found in bacterial and fungal phospholipases. It enables the liberation of fatty acids and lysophospholipid by hydrolyzing the 2-ester bond of 1,2-diacyl-3-sn-phosphoglycerides. The domain adopts an alpha-helical secondary structure, consisting of five alpha-helices and two helical segments.[2]
References
- ^ Matoba Y, Katsube Y, Sugiyama M (May 2002). "The crystal structure of prokaryotic phospholipase A2". J. Biol. Chem. 277 (22): 20059–69. doi:10.1074/jbc.M200263200. PMID 11897785.
- ^ Katsube Y, Sugiyama M, Matoba Y (2002). "The crystal structure of prokaryotic phospholipase A2". J. Biol. Chem. 277 (22): 20059–69. doi:10.1074/jbc.M200263200. PMID 11897785.