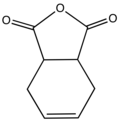
Tetrahydrophthalic anhydride
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(3aR,7aS)-3a,4,7,7a-Tetrahydro-2-benzofuran-1,3-dione | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.012.098 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2698 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H8O3 | |
| Molar mass | 152.149 g·mol−1 |
| Appearance | white or colorless solid |
| Melting point | 97–103 °C (207–217 °F; 370–376 K) |
| Hazards | |
| GHS labelling: | |
| |
| Danger | |
| H317, H318, H334, H412 | |
| P261, P272, P273, P280, P285, P302+P352, P304+P341, P305+P351+P338, P310, P321, P333+P313, P342+P311, P363, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Tetrahydrophthalic anhydride is an organic compound with the formula C6H8C2O3. The compound exists as two isomers, this article being focused on the more common cis isomer. It is a white solid that is soluble in organic solvents.
Preparation and derivatives
Tetrahydrophthalic anhydride, the cis isomer, is prepared by the Diels-Alder reaction of butadiene and maleic anhydride.[1]
Tetrahydrophthalic anhydride is a precursor to other compounds including the dicarboxylic acid tetrahydrophthalic acid as well the tetrahydrophthalimide, which is a precursor to the fungicide Captan. It is also a precursor to 1,2,3,4-butanetetracarboxylic acid.[2]
References
- ^ Arthur C. Cope; Elbert C. Herrick (1950). "cis-Δ4-Tetrahydrophthalic Anhydride". Org. Synth. 50: 93. doi:10.15227/orgsyn.030.0093.
- ^ Nagao, R.; Marumo, F.; Saito, Y.; Asahara, T. (1971). "The Crystal Structure of Butane-1,2,3,4-tetracarboxylic Dianhydride". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 27 (3): 569–572. Bibcode:1971AcCrB..27..569N. doi:10.1107/s0567740871002577.