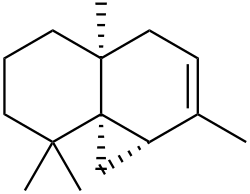
Thujopsene
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(1aS,4aS,8aS)-2,4a,8,8-Tetramethyl-1,1a,4,4a,5,6,7,8-octahydrocyclopropa[d]naphthalene | |
| Other names
Sesquichamene; Thujopsen; Widdrene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.006.753 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H24 | |
| Molar mass | 204.357 g·mol−1 |
| Density | 0.936 g/mL (20 °C)[1] |
| Boiling point | 258 to 260 °C (496 to 500 °F; 531 to 533 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Thujopsene is a natural chemical compound, classified as a sesquiterpene, with the molecular formula C15H24.
Thujopsene is found in the essential oil of a variety of conifers,[2] in particular Juniperus cedrus and Thujopsis dolabrata in which it comprises around 2.2% of the weight of the heartwood.[3]
Biosynthesis
Thujopsene is biosynthesized from farnesyl pyrophosphate (FPP):[4]
References
- ^ a b "(−)-Thujopsene". Sigma-Aldrich.
- ^ Erdtman, H.; Norin, T. (1960). "Structure of thujopsene and hinokiic acid from coniferous wood". Chemistry and Industry (22): 622–623.
- ^ Runeburg, Jarl; Gramstad, Thor; Larsson, Lennart; Dodson, R. M. (1960). "The Chemistry of the Natural Order Cupressales XXX. Constituents of Juniperus cedrus L.". Acta Chemica Scandinavica. 14: 1991–1994. doi:10.3891/acta.chem.scand.14-1991.
- ^ J. Mann; et al. (1994). Natural Products: their chemistry and biological significance. Longman Scientific & Technical. ISBN 978-0582060098.
