Tin(II) iodide
Sn2+ (I−)2
| |
| Names | |
|---|---|
| IUPAC name
tin(II) iodide
| |
| Other names
stannous iodide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.594 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| I2Sn | |
| Molar mass | 372.519 g·mol−1 |
| Appearance | red to red-orange solid |
| Density | 5.28 g/cm3 |
| Melting point | 320 °C (608 °F; 593 K) |
| Boiling point | 714 °C (1,317 °F; 987 K) |
| 0.98 g/100 g | |
| Related compounds | |
Other anions
|
tin dichloride, tin(II) bromide |
Other cations
|
lead(II) iodide |
Related compounds
|
tin tetraiodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Tin(II) iodide, also known as stannous iodide, is the inorganic compound with the formula SnI2. It is a red-orange solid. It reacts with iodine to give tin(IV) iodide.[1]
Tin(II) iodide can be synthesised by heating metallic tin with a mixture iodine in 2 M hydrochloric acid.[2][1]
- Sn + I2 → SnI2
Structure
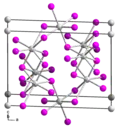
It crystallizes in a unique motif. According to X-ray crystallography, some Sn(II) centers are bound to six iodide ligands others Sn(II) sites are distorted.[3]
References
- ^ a b Stolzenberg, Alan M. (2014). "Tin(II) Iodide". Inorganic Syntheses: Volume 36. Vol. 36. p. 240. doi:10.1002/9781118744994.ch44. ISBN 978-1-118-74487-1.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 380–381. doi:10.1016/C2009-0-30414-6. ISBN 978-0-08-037941-8.
- ^ Howie, R. A.; Moser, W.; Trevena, I. C. (1972). "The Crystal Structure of Tin(II) Iodide". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 28 (10): 2965–2971. Bibcode:1972AcCrB..28.2965H. doi:10.1107/S0567740872007290.