Tumor-associated glycoprotein 72
Tumor-associated glycoprotein 72 (TAG-72) is a glycoprotein that appears on the surface of many cancer cells, including those from the ovary,[1][2][3] breast, colon,[4] lung, and pancreatic cancers.[5][6] TAG-72 is a mucin-like molecule with a molar mass of over 1000 kDa, and is classified as a tumor-associated glycoprotein.[7]
Discovery
Researchers identified Tumor-associated glycoprotein 72 (TAG-72) in the mid-1980s during the development of the Monoclonal antibody B72.3. These antibodies selectively bound to a high-molecular-weight glycoprotein found on various Carcinoma cells. Later studies confirmed TAG-72 as a Mucin-like molecule with significant Glycosylation, which adds to its high molecular weight.[8] This discovery has supported advancements in cancer diagnostics and therapeutics, especially those targeting TAG-72-expressing tumors.[9]
Structure
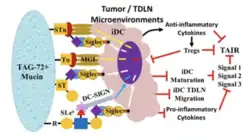
TAG-72 is a high-molecular-weight glycoprotein(>1,000 Kda), primarily expressed on the surface of various Adenocarcinomas. Its structure is features extensive O-linked glycosylation,[9] which gives it a mucin-like configuration.[9][10][8] The glycosylation patterns of TAG-72 include tumor-associated carbohydrate antigens such assialyl-Tn (STn) and Thomsen–Friedenreich antigens, which are contribute to tumor progression and metastasis.[11] These carbohydrate epitopes serve as binding sites for monoclonal antibodies like B72.3 and CC49, enabling targeted cancer detection and treatment.[9]
Pathogenic mechanism
TAG-72 rarely appears in normal adult tissues but is highly present in malignant epithelial cells, which makes it a Tumor-specific antigen.[8] It plays a role in:
- Tumor Progression: TAG-72 forms part of the mucinous barrier that shields tumor cells from immune recognition.[12]
- Cell Adhesion and Metastasis: Its Glycosylation influences how tumor cells interact with the extracellular matrix, facilitating metastasis.[13]
Clinical applications
1. Tumor Marker (CA 72-4 Assay)
TAG-72 is commonly measured with radioimmunoassays like CA 72-4,[14] which uses the monoclonal antibodies indium (111In) satumomab pendetide and iodine (125I) minretumomab.[15][16][17][18][19] This assay has a good specificity for gastric cancer, with a correlation to the neoplasia's extension. It is used for:
- Cancer diagnosis and staging[20]
- Monitoring recurrence and therapy effectiveness
- Targeted Cancer Therapies[9]
Since TAG-72 is tumor-specific, it is a promising target for immunotherapy and antibody-drug conjugates:
- Monoclonal Antibody Therapy:
- Minretumomab (CC49) has been studied for targeting TAG-72 in solid tumors.[21]
- Anatumomab mafenatox is an anti-TAG-72 antibody conjugated with toxins for cancer treatment.[22]
- CAR-T Cell Therapy: CAR-T cells engineered to recognize TAG-72 have been tested in ovarian and colorectal cancer.[23]
Cancer association
TAG-72 is mainly found in epithelial-derived malignancies, including:
Gastrointestinal
- Colorectal cancer: TAG-72 is overexpressed in colorectal Adenocarcinoma, and serum levels often reflect tumor stage and prognosis[24]
- Gastric cancer: CA 72-4, an immunoassay detecting TAG-72, is widely used for diagnosing and monitoring gastric cancer.[25]
- Pancreatic cancer: TAG-72 levels have helped in diagnosing late stage pancreatic cancers.[26]
Gynecological
- Ovarian cancer: TAG-72 expression correlates with tumor stage and patient prognosis.[27]
Other cancers
- Lung cancer: TAG-72 is present in a subset of non-small cell lung carcinomas.[28]
- Breast cancer: Although less specific, TAG-72 has been detected in certain aggressive breast cancer subtypes.[29]
References
- ^ Ponnusamy MP, Venkatraman G, Singh AP, Chauhan SC, Johansson SL, Jain M, et al. (2007-06-28). "Expression of TAG-72 in ovarian cancer and its correlation with tumor stage and patient prognosis". Cancer Letters. 251 (2): 247–257. doi:10.1016/j.canlet.2006.11.025. PMID 17210225.
- ^ Murad JP, Kozlowska AK, Lee HJ, Ramamurthy M, Chang WC, Yazaki P, et al. (2018-11-19). "Effective Targeting of TAG72+ Peritoneal Ovarian Tumors via Regional Delivery of CAR-Engineered T Cells". Frontiers in Immunology. 9: 2268. doi:10.3389/fimmu.2018.02268. PMC 6254427. PMID 30510550.
- ^ Shu R, Evtimov VJ, Hammett MV, Nguyen NN, Zhuang J, Hudson PJ, et al. (2021-01-16). "Engineered CAR-T cells targeting TAG-72 and CD47 in ovarian cancer". Molecular Therapy Oncolytics. 20: 325–341. doi:10.1016/j.omto.2021.01.002. PMC 7868933. PMID 33614914.
- ^ Hege KM, Bergsland EK, Fisher GA, Nemunaitis JJ, Warren RS, McArthur JG, et al. (2017-03-21). "Safety, tumor trafficking and immunogenicity of chimeric antigen receptor (CAR)-T cells specific for TAG-72 in colorectal cancer". Journal for Immunotherapy of Cancer. 5 22. doi:10.1186/s40425-017-0222-9. PMC 5360066. PMID 28344808.
- ^ TAG-72 antigen entry in the public domain NCI Dictionary of Cancer Terms
- ^ Scott AM, Wolchok JD, Old LJ (Mar 2012). "Antibody therapy of cancer". Nature Reviews. Cancer. 12 (4): 278–287. doi:10.1038/nrc3236. PMID 22437872. S2CID 205469234.
- ^ Sheer DG, Schlom J, Cooper HL (Dec 1988). "Purification and Composition of the Human Tumor-associated Glycoprotein (TAG-72) Defined by Monoclonal Antibodies CC49 and B72.3". Cancer Research. 48 (23): 6811–6818. PMID 3180090.
- ^ a b c Chopra A (2004), "99mTc-Labeled tumor-associated glycoprotein 72 binding peptides A2-6 and A3-10", Molecular Imaging and Contrast Agent Database (MICAD), Bethesda (MD): National Center for Biotechnology Information (US), PMID 22049573, retrieved 2025-04-19
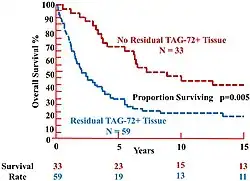
- ^ a b c d e f Hitchcock CL, Povoski SP, Mojzisik CM, Martin EJ (2021-12-07). "Survival Advantage Following TAG-72 Antigen-Directed Cancer Surgery in Patients With Colorectal Carcinoma: Proposed Mechanisms of Action". Frontiers in Oncology. 11: 731350. doi:10.3389/fonc.2021.731350. ISSN 2234-943X. PMC 8688248. PMID 34950576.
- ^ Riley NM, Wen RM, Bertozzi CR, Brooks JD, Pitteri SJ (2023). "Measuring the multifaceted roles of mucin-domain glycoproteins in cancer". Advances in Cancer Research. 157: 83–121. doi:10.1016/bs.acr.2022.09.001. ISBN 978-0-323-99177-3. ISSN 2162-5557. PMC 10582998. PMID 36725114.
- ^ Rajesh C, Radhakrishnan P (2023-01-06). "The (Sialyl) Tn antigen: Contributions to immunosuppression in gastrointestinal cancers". Frontiers in Oncology. 12: 1093496. doi:10.3389/fonc.2022.1093496. ISSN 2234-943X. PMC 9852904. PMID 36686742.
- ^ Bhatia R, Gautam SK, Cannon A, Thompson C, Hall BR, Aithal A, et al. (June 2019). "Cancer-associated mucins: role in immune modulation and metastasis". Cancer and Metastasis Reviews. 38 (1–2): 223–236. doi:10.1007/s10555-018-09775-0. ISSN 1573-7233. PMC 6614013. PMID 30618016.
- ^ Laubli H, Borsig L (2019). "Altered Cell Adhesion and Glycosylation Promote Cancer Immune Suppression and Metastasis". Frontiers in Immunology. 10: 2120. doi:10.3389/fimmu.2019.02120. ISSN 1664-3224. PMC 6743365. PMID 31552050.
- ^ Guadagni F, Roselli M, Amato T, Cosimelli M, Perri P, Casale V, et al. (1992-03-01). "CA 72-4 measurement of tumor-associated glycoprotein 72 (TAG-72) as a serum marker in the management of gastric carcinoma". Cancer Research. 52 (5): 1222–1227. ISSN 0008-5472. PMID 1737383.
- ^ Louhimo J, Alfthan H, Stenman UH, Haglund C (2004). "Serum HCG beta and CA 72-4 are stronger prognostic factors than CEA, CA 19-9 and CA 242 in pancreatic cancer". Oncology. 66 (2): 126–131. doi:10.1159/000077438. PMID 15138364. S2CID 25798287.
- ^ Louhimo J, Carpelan-Holmström M, Alfthan H, Stenman UH, Järvinen HJ, Haglund C (October 2002). "Serum HCG beta, CA 72-4 and CEA are independent prognostic factors in colorectal cancer". International Journal of Cancer. 101 (6): 545–548. doi:10.1002/ijc.90009. PMID 12237895.
- ^ Louhimo J, Kokkola A, Alfthan H, Stenman UH, Haglund C (October 2004). "Preoperative hCGbeta and CA 72-4 are prognostic factors in gastric cancer". International Journal of Cancer. 111 (6): 929–933. doi:10.1002/ijc.20321. PMID 15300805. S2CID 24698852.
- ^ Mattar R, Alves de Andrade CR, DiFavero GM, Gama-Rodrigues JJ, Laudanna AA (2002). "Preoperative serum levels of CA 72-4, CEA, CA 19-9, and alpha-fetoprotein in patients with gastric cancer". Revista do Hospital das Clinicas. 57 (3): 89–92. doi:10.1590/s0041-87812002000300001. PMID 12118264.
- ^ Guadagni F, Roselli M, Cosimelli M, Spila A, Cavaliere F, Tedesco M, et al. (November 1996). "Correlation between tumor-associated glycoprotein 72 mucin levels in tumor and serum of colorectal patients as measured by the quantitative CA 72-4 immunoassay". Cancer Research. 56 (22): 5293–5298. PMID 8912871.
- ^ Guadagni F, Roselli M, Ferroni P, Amato T, Colcher D, Greiner JW, et al. (1991). "Clinical evaluation of the new tumor marker TAG-72". Anticancer Research. 11 (4): 1389–1394. ISSN 0250-7005. PMID 1746895.
- ^ "Anti-TAG-72 [Minretumomab (CC49 )]". Absolute Antibody. Retrieved 2025-04-19.
- ^ "Anti-Human TAG-72 Recombinant Antibody(Anatumomab Mafenatox) - KMD Bioscience-Nanobody Discovery Platform, Protein Expression Systems, and CRO Service Supplier". www.kmdbioscience.com. Retrieved 2025-04-19.
- ^ Shu R, Evtimov VJ, Hammett MV, Nguyen NN, Zhuang J, Hudson PJ, et al. (2021-03-26). "Engineered CAR-T cells targeting TAG-72 and CD47 in ovarian cancer". Molecular Therapy Oncolytics. 20: 325–341. doi:10.1016/j.omto.2021.01.002. ISSN 2372-7705. PMC 7868933. PMID 33614914.
- ^ Cho J, Kim KM, Kim HC, Lee WY, Kang WK, Park YS, et al. (January 2019). "The prognostic role of tumor associated glycoprotein 72 (TAG-72) in stage II and III colorectal adenocarcinoma". Pathology, Research and Practice. 215 (1): 171–176. doi:10.1016/j.prp.2018.10.024. ISSN 1618-0631. PMID 30466765.
- ^ Xu Y, Zhang P, Zhang K, Huang C (2021-12-01). "The application of CA72-4 in the diagnosis, prognosis, and treatment of gastric cancer". Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 1876 (2) 188634. BBA. doi:10.1016/j.bbcan.2021.188634. ISSN 0304-419X. PMID 34656687.
- ^ Pasquali C, Sperti C, D'Andrea AA, Costantino V, Filipponi C, Pedrazzoli S (June 1994). "Clinical value of serum TAG-72 as a tumor marker for pancreatic carcinoma. Comparison with CA 19-9". International Journal of Pancreatology. 15 (3): 171–177. doi:10.1007/BF02924191. ISSN 0169-4197. PMID 7930777.
- ^ Ponnusamy MP, Venkatraman G, Singh AP, Chauhan SC, Johansson SL, Jain M, et al. (2007-06-28). "Expression of TAG-72 in ovarian cancer and its correlation with tumor stage and patient prognosis". Cancer Letters. 251 (2): 247–257. doi:10.1016/j.canlet.2006.11.025. ISSN 0304-3835. PMID 17210225.
- ^ Chen L, Wang Y, Liu X, Dou S, Liu G, Hnatowich DJ, et al. (2008-12-08). "A new TAG-72 cancer marker peptide identified by phage display". Cancer Letters. 272 (1): 122–132. doi:10.1016/j.canlet.2008.07.009. ISSN 1872-7980. PMC 2680929. PMID 18723274.
- ^ Galietta A, Pizzi C, Pettinato G, Limite G, Sgambato A, Lamberti M, et al. (2002). "Differential TAG-72 epitope expression in breast cancer and lymph node metastases: a marker of a more aggressive phenotype". Oncology Reports. 9 (1): 135–140. ISSN 1021-335X. PMID 11748471.
![]() This article incorporates public domain material from Dictionary of Cancer Terms. U.S. National Cancer Institute.
This article incorporates public domain material from Dictionary of Cancer Terms. U.S. National Cancer Institute.