Alalevonadifloxacin
 | |
| Clinical data | |
|---|---|
| Trade names | Emrok O |
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
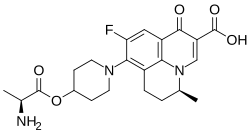
| Formula | C22H26FN3O5 |
| Molar mass | 431.464 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Alalevonadifloxacin (trade name Emrok O) is an antibiotic of the fluoroquinolone class.[1] It is a prodrug of levonadifloxacin with increased oral bioavailability.[2] In India, it is approved for the treatment of infections with Gram-positive bacteria.[3]
References
- ^ Saseedharan S, Dubey D, Singh RK, Zirpe K, Choudhuri AH, Mukherjee DN, et al. (2024). "Treatment challenges in the management of difficult-to-treat gram-positive infections: A consensus view apropos therapeutic role of novel anti-MRSA antibiotics, levonadifloxacin (IV) and alalevonadifloxacin (oral)". Indian Journal of Medical Microbiology. 47 100528. doi:10.1016/j.ijmmb.2024.100528. PMID 38228227.
- ^ Bhawsar S, Kale R, Deshpande P, Yeole R, Bhagwat S, Patel M (December 2021). "Design and synthesis of an oral prodrug alalevonadifloxacin for the treatment of MRSA infection". Bioorganic & Medicinal Chemistry Letters. 54 128432. doi:10.1016/j.bmcl.2021.128432. PMID 34757217.
- ^ "Alalevonadifloxacin". AdisInsight. Springer Nature Switzerland AG.