Secnidazole
 | |
| Clinical data | |
|---|---|
| Trade names | Solosec |
| Other names | PM-185184; RP-14539; SYM-1219 |
| AHFS/Drugs.com | International Drug Names |
| License data |
|
| Routes of administration | Oral[1] |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.020.123 |
| Chemical and physical data | |
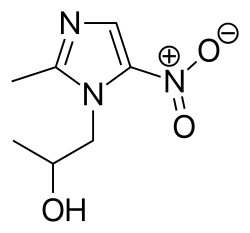
| Formula | C7H11N3O3 |
| Molar mass | 185.183 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Secnidazole (trade names Flagentyl, Sindose, Secnil, Solosec) is a nitroimidazole anti-infective used to treat bacterial vaginosis and trichomoniasis.[2][1] It is taken orally.[1]
Structurally it actually methyl-metronidazole. Effectiveness in the treatment of dientamoebiasis has been reported.[3] It has also been tested against Atopobium vaginae.[4]
In the United States, secnidazole is FDA-approved for the treatment of bacterial vaginosis and trichomoniasis in adult women.[2][5] It was approved in the United States in 2017.[1]
References
- ^ a b c d fda.gov
- ^ a b "Evofem Biosciences". AdisInsight. 24 February 2025. Retrieved 26 February 2025.
- ^ Girginkardeşler N, Coşkun S, Cüneyt Balcioğlu I, Ertan P, Ok UZ (February 2003). "Dientamoeba fragilis, a neglected cause of diarrhea, successfully treated with secnidazole". Clinical Microbiology and Infection. 9 (2): 110–3. doi:10.1046/j.1469-0691.2003.00504.x. PMID 12588330.
- ^ De Backer E, Dubreuil L, Brauman M, Acar J, Vaneechoutte M (May 2010). "In vitro activity of secnidazole against Atopobium vaginae, an anaerobic pathogen involved in bacterial vaginosis". Clinical Microbiology and Infection. 16 (5): 470–2. doi:10.1111/j.1469-0691.2009.02852.x. PMID 19548924.
- ^ Muzny CA, Van Gerwen OT (April 2022). "Secnidazole for Trichomoniasis in Women and Men". Sex Med Rev. 10 (2): 255–262. doi:10.1016/j.sxmr.2021.12.004. PMC 11019772. PMID 35153156. S2CID 246755406.