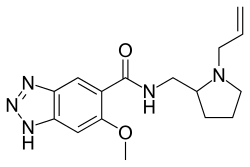
Alizapride
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, IM, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 3 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.056.082 |
| Chemical and physical data | |
| Formula | C16H21N5O2 |
| Molar mass | 315.377 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Alizapride (Litican, Plitican, Superan, Vergentan) is a dopamine antagonist with prokinetic and antiemetic effects used in the treatment of nausea and vomiting, including postoperative nausea and vomiting. It is structurally related to metoclopramide and other benzamides.[1]
Mechanism
Alizapride acts on the vomiting center by blocking D2 dopamine receptors.[2]
Since alizapride is able to cross the blood-brain barrier, adverse effects may include temporary extrapyramidal motor disorders such as acute dystonia and dyskinesia.[3]
It has a plasma half-life of 3 hours.[3]
Synthesis
The synthesis of Alizapride happens in multiple steps:[4]
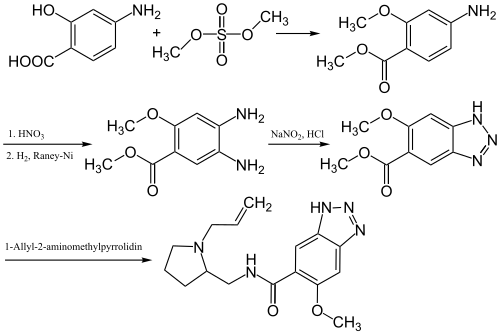
4-Aminosalicylic acid is first methylated using dimethyl sulfate. A nitro group is then introduced that is reduced using Raney nickel to afford an amino group. The two amino groups are then closed to a triazole ring using sodium nitrite and hydrochloric acid. This is then condensed with 1-allyl-2-aminomethylpyrrolidine to afford Alizapride.
References
- ^ Ballatori E, Roila F (September 2003). "Impact of nausea and vomiting on quality of life in cancer patients during chemotherapy". Health and Quality of Life Outcomes. 1: 46. doi:10.1186/1477-7525-1-46. PMC 212194. PMID 14521717.
- ^ Online GL (October 17, 2016). "Anwendung, Wirkung, Nebenwirkungen". Gelbe Liste Online (in German). Retrieved April 30, 2025.
- ^ a b Geisslinger G, Menzel S, Gundermann T, Roth P (2020). Mutschler Arzneimittelwirkungen (11 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. p. 580. ISBN 978-3-8047-3663-4.
- ^ Kleemann A, Engel J, Kutscher B, Reichert D (2014). Pharmaceutical Substances, 5th Edition: Syntheses, Patents and Applications of the most relevant APIs. Georg Thieme Verlag. p. 41. ISBN 978-3-13-179525-0.