Cycloheptyl CP 55,940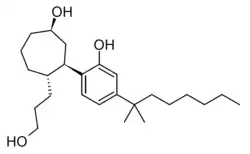 |
|
(1R,3R,4R)-3-[4-(1,1-dimethylheptyl)-2-hydroxyphenyl]-4-(3-hydroxypropyl)cycloheptan-1-ol
|
| CAS Number | |
|---|
|
| Formula | C25H42O3 |
|---|
| Molar mass | 390.608 g·mol−1 |
|---|
| 3D model (JSmol) | |
|---|
O[C@H]1C[C@H]([C@H](CCCO)CCC1)c1ccc(cc1O)C(C)(C)CCCCCC
|
InChI=InChI=1S/C25H42O3/c1-4-5-6-7-15-25(2,3)20-13-14-22(24(28)17-20)23-18-21(27)12-8-10-19(23)11-9-16-26/h13-14,17,19,21,23,26-28H,4-12,15-16,18H2,1-3H3/t19-,21+,23+/m0/s1 Key:JYVBHJZDNJZVAK-XKCSPQBFSA-N
|
Cycloheptyl CP 55,940 is a synthetic cannabinoid related to CP 55,940 but is a ring-expanded homologue with a cycloheptyl ring in place of the cyclohexyl ring. It was first synthesized by Pfizer in the 1980s.[1][2] It falls outside the definition of a "cyclohexylphenol derivative" since it does not have a cyclohexyl ring. Cycloheptyl CP 55,940 has similar potency to CP 55,940 itself, with an ED50 of 0.06 mg/kg in animal studies.[3]
See also
References
- ^ Melvin LS, Milne GM, Johnson MR, Subramaniam B, Wilken GH, Howlett AC (November 1993). "Structure-activity relationships for cannabinoid receptor-binding and analgesic activity: studies of bicyclic cannabinoid analogs". Molecular Pharmacology. 44 (5): 1008–1015. doi:10.1016/S0026-895X(25)13256-2. PMID 8246904.
- ^ Melvin LS, Milne GM, Johnson MR, Wilken GH, Howlett AC (November 1995). "Structure-activity relationships defining the ACD-tricyclic cannabinoids: cannabinoid receptor binding and analgesic activity". Drug Design and Discovery. 13 (2): 155–166. PMID 8872458.
- ^ US 4371720, Johnson MR, Melvin LS, "2-Hydroxy-4-(substituted) phenyl cycloalkanes and derivatives.", issued 1 February 1983, assigned to Pfizer Inc.
|
|---|
Phytocannabinoids
(comparison) | | Cannabibutols | |
|---|
| Cannabichromenes | |
|---|
| Cannabicyclols | |
|---|
| Cannabidiols | |
|---|
| Cannabielsoins | |
|---|
| Cannabigerols | |
|---|
| Cannabiphorols | |
|---|
| Cannabinols |
- CBN
- CBNA
- CBN-C1
- CBN-C2
- CBN-C4
- CBNM
- CBND
- CBNP
- CBVD
|
|---|
| Cannabitriols | |
|---|
| Cannabivarins | |
|---|
| Delta-3-tetrahydrocannabinols | |
|---|
| Delta-4-tetrahydrocannabinols | |
|---|
| Delta-7-tetrahydrocannabinols | |
|---|
| Delta-8-tetrahydrocannabinols | |
|---|
| Delta-9-tetrahydrocannabinols | |
|---|
| Delta-10-Tetrahydrocannabinols | |
|---|
| Delta-11-Tetrahydrocannabinols | |
|---|
| Miscellaneous cannabinoids | |
|---|
| Active metabolites | |
|---|
|
|---|
| Endocannabinoids | |
|---|
Synthetic
cannabinoid
receptor
agonists /
neocannabinoids | Classical cannabinoids
(dibenzopyrans) | |
|---|
Non-classical
cannabinoids | |
|---|
| Adamantoylindoles | |
|---|
| Benzimidazoles | |
|---|
| Benzoylindoles | |
|---|
| Cyclohexylphenols | |
|---|
| Eicosanoids | |
|---|
Indazole-3-
carboxamides | |
|---|
| Indole-3-carboxamides | |
|---|
| Indole-3-carboxylates | |
|---|
| Naphthoylindazoles | |
|---|
| Naphthoylindoles | |
|---|
| Naphthoylpyrroles | |
|---|
| Naphthylmethylindenes | |
|---|
| Naphthylmethylindoles | |
|---|
| Phenylacetylindoles | |
|---|
| Pyrazolecarboxamides | |
|---|
Tetramethylcyclo-
propanoylindazoles | |
|---|
Tetramethylcyclo-
propanoylindoles | |
|---|
| Others | |
|---|
|
|---|
| Allosteric CBRTooltip Cannabinoid receptor ligands | |
|---|
Endocannabinoid
enhancers
(inactivation inhibitors) | |
|---|
Anticannabinoids
(antagonists/inverse
agonists/antibodies) | |
|---|
|
