Δ7-Tetrahydrocannabinol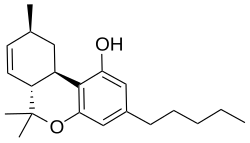 |
|
(6aR,9S,10aR)-6,6,9-trimethyl-3-pentyl-6a,9,10,10a-tetrahydrobenzo[c]chromen-1-ol
|
| CAS Number | |
|---|
| PubChem CID | |
|---|
| ChemSpider | |
|---|
| UNII | |
|---|
| KEGG | |
|---|
| CompTox Dashboard (EPA) | |
|---|
|
| Formula | C21H30O2 |
|---|
| Molar mass | 314.469 g·mol−1 |
|---|
| 3D model (JSmol) | |
|---|
CCCCCC1=CC(=C2[C@@H]3C[C@@H](C=C[C@H]3C(OC2=C1)(C)C)C)O
|
InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h9-10,12-14,16-17,22H,5-8,11H2,1-4H3/t14-,16-,17-/m1/s1 Key:WWYMYGIVLCKTBL-DJIMGWMZSA-N
|
Δ7-Tetrahydrocannabinol (Delta-7-THC, Δ7-THC; alternatively numbered as Δ5-tetrahydrocannabinol, Δ5-THC) is a synthetic isomer of tetrahydrocannabinol. The (6aR,9S,10aR)-Δ7-THC epimer is only slightly less potent than Δ9-THC itself, while the (9R) epimer is much less potent.[1][2]
See also
References
- ^ Mechoulam R, Ben-Zvi Z, Varconi H, Samuelov Y (1973). "Cannabinoid rearrangements: Synthesis of Δ5-tetrahydrocannabinol". Tetrahedron. 29 (11): 1615–1619. doi:10.1016/S0040-4020(01)83406-2.
- ^ Huffman JW, Banner WK, Zoorob GK, Joyner HH, Reggio PH, Martin BR, Compton DR (1995). "Stereoselective synthesis of the epimeric Δ7-tetrahydrocannabinols". Tetrahedron. 51 (4): 1017–1032. doi:10.1016/0040-4020(94)00995-7.
|
|---|
Phytocannabinoids
(comparison) | | Cannabibutols | |
|---|
| Cannabichromenes | |
|---|
| Cannabicyclols | |
|---|
| Cannabidiols | |
|---|
| Cannabielsoins | |
|---|
| Cannabigerols | |
|---|
| Cannabiphorols | |
|---|
| Cannabinols |
- CBN
- CBNA
- CBN-C1
- CBN-C2
- CBN-C4
- CBNM
- CBND
- CBNP
- CBVD
|
|---|
| Cannabitriols | |
|---|
| Cannabivarins | |
|---|
| Delta-3-tetrahydrocannabinols | |
|---|
| Delta-4-tetrahydrocannabinols | |
|---|
| Delta-7-tetrahydrocannabinols | |
|---|
| Delta-8-tetrahydrocannabinols | |
|---|
| Delta-9-tetrahydrocannabinols | |
|---|
| Delta-10-Tetrahydrocannabinols | |
|---|
| Delta-11-Tetrahydrocannabinols | |
|---|
| Miscellaneous cannabinoids | |
|---|
| Active metabolites | |
|---|
|
|---|
| Endocannabinoids | |
|---|
Synthetic
cannabinoid
receptor
agonists /
neocannabinoids | Classical cannabinoids
(dibenzopyrans) | |
|---|
Non-classical
cannabinoids | |
|---|
| Adamantoylindoles | |
|---|
| Benzimidazoles | |
|---|
| Benzoylindoles | |
|---|
| Cyclohexylphenols | |
|---|
| Eicosanoids | |
|---|
Indazole-3-
carboxamides | |
|---|
| Indole-3-carboxamides | |
|---|
| Indole-3-carboxylates | |
|---|
| Naphthoylindazoles | |
|---|
| Naphthoylindoles | |
|---|
| Naphthoylpyrroles | |
|---|
| Naphthylmethylindenes | |
|---|
| Naphthylmethylindoles | |
|---|
| Phenylacetylindoles | |
|---|
| Pyrazolecarboxamides | |
|---|
Tetramethylcyclo-
propanoylindazoles | |
|---|
Tetramethylcyclo-
propanoylindoles | |
|---|
| Others | |
|---|
|
|---|
| Allosteric CBRTooltip Cannabinoid receptor ligands | |
|---|
Endocannabinoid
enhancers
(inactivation inhibitors) | |
|---|
Anticannabinoids
(antagonists/inverse
agonists/antibodies) | |
|---|
|
