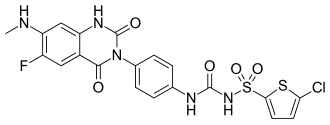
Elinogrel
 | |
| Clinical data | |
|---|---|
| Other names | PRT-060128 |
| Routes of administration | By mouth, IV |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Mainly unchanged, ~15% N-demethylation[1] |
| Excretion | Urine, faeces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H15ClFN5O5S2 |
| Molar mass | 523.94 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Elinogrel (INN,[2] USAN) was an experimental antiplatelet drug acting as a P2Y12 inhibitor. Similarly to ticagrelor and in contrast to clopidogrel, elinogrel was a reversible inhibitor that acted fast and short (for about 12 hours), and it was not a prodrug but pharmacologically active itself. The substance was used in form of its potassium salt, intravenously for acute treatment and orally for long-term treatment.[3] Development was terminated in 2012.
History
The substance was originally developed by Portola Pharmaceuticals, with Phase II clinical trials conducted around 2008–2011.[4] In February 2009, Novartis bought worldwide rights to develop it further, intending to conduct Phase III studies and commercialise the drug.[5] The development of the drug was terminated in January 2012 by Novartis.[6]
References
- ^ Siller-Matula JM, Krumphuber J, Jilma B (February 2010). "Pharmacokinetic, pharmacodynamic and clinical profile of novel antiplatelet drugs targeting vascular diseases". British Journal of Pharmacology. 159 (3): 502–17. doi:10.1111/j.1476-5381.2009.00555.x. PMC 2828016. PMID 20050853.
- ^ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 63" (PDF). World Health Organization. pp. 50–1. Retrieved 1 December 2016.
- ^ Gurbel PA, Kereiakes D, Tantry US (November 2010). "Elinogrel potassium: Receptor antagonist antiplatelet therapy". Drugs of the Future. 35 (11): 885–92. doi:10.1358/dof.2010.35.11.1529823. S2CID 79785538.
- ^ Michelson AD (2011). "Advances in antiplatelet therapy". Hematology. American Society of Hematology. Education Program. 2011: 62–9. doi:10.1182/asheducation-2011.1.62. PMID 22160013.
- ^ "Novartis gains worldwide rights to elinogrel, a Phase II anti-clotting compound with potential to reduce risk of heart attack". Insciences. Archived from the original on 2014-07-14.
- ^ "Novartis drops elinogrel outright". BioPortfolio. Archived from the original on 2012-07-29.