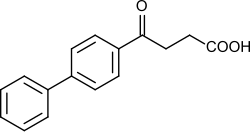
Fenbufen
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.048.148 |
| Chemical and physical data | |
| Formula | C16H14O3 |
| Molar mass | 254.285 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 186 °C (367 °F) |
| |
| |
| | |
Fenbufen is a nonsteroidal anti-inflammatory drug used to treat pain.[1]
Fenbufen is a member of the propionic acid derivatives class of drugs.[2]
It was introduced by American Cyanamid under the trade name Lederfen in the 1980s. Due to liver toxicity, it was withdrawn from markets in the developed world in 2010.[3][4]: 370, 383–384
As of 2015 it was available in Taiwan and Thailand under several brand names.[5]
Preparation
Fenbufen can be synthesized by acylation of biphenyl with succinic anhydride under Friedel-Crafts conditions.[6]
References
- ^ Moore RA, Derry S, McQuay HJ (October 2009). "Single dose oral fenbufen for acute postoperative pain in adults". The Cochrane Database of Systematic Reviews. 2009 (4): CD007547. doi:10.1002/14651858.CD007547.pub2. PMC 4175557. PMID 19821427.
- ^ Brogden RN (1986). "Non-steroidal anti-inflammatory analgesics other than salicylates". Drugs. 32 (Suppl 4): 27–45. doi:10.2165/00003495-198600324-00004. PMID 3552584. S2CID 25471102.
- ^ "Deleted products 2010". Monthly Index of Medical Specialities (MIMS). Haymarket Media Group Ltd.
- ^ Lewis JH, Stine JG (2013). "Nonsteroidal Antiinflammatory Drugs and Leukotriene Receptor Antagonists. Chapter 22". In Kaplowitz N, DeLeve LD (eds.). Drug-Induced Liver Disease (3rd ed.). Academic Press. ISBN 978-0-12-387818-2.
- ^ "International listings for fenbufen". Drugs.com. Retrieved 25 June 2015.
- ^ Castillo R, Suárez-Herrera M, Aparicio M, Hernández-Lui F, Hernández A (October 1995). "An Improved Synthesis of Fenbulen". Organic Preparations and Procedures International. 27 (5): 550–552. doi:10.1080/00304949509458497.