Fourphit
 | |
| Clinical data | |
|---|---|
| Other names | 4-Isothiocyanato-PCP |
| Drug class | Irreversible dopamine transporter blocker; Reversible NMDA receptor antagonist; Psychostimulant antagonist |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
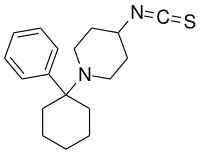
| Formula | C18H24N2S |
| Molar mass | 300.46 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Fourphit, also known as 4-isothiocyanato-PCP, is an irreversible dopamine transporter (DAT) blocker and a reversible NMDA receptor antagonist.[1][2] It blocks the binding of methylphenidate to the DAT in vitro,[1] though apparently not in vivo.[2] In any case, the drug reduces the stimulant-like effects of cocaine in animals,[2] whilst producing mostly negligible behavioral effects itself.[2] Fourphit is an acylating derivative of phencyclidine (PCP) and a positional isomer of metaphit (3-isothiocyanato-PCP).[1]
See also
References
- ^ a b c Schweri MM, Thurkauf A, Mattson MV, Rice KC (June 1992). "Fourphit: a selective probe for the methylphenidate binding site on the dopamine transporter". J Pharmacol Exp Ther. 261 (3): 936–942. doi:10.1016/S0022-3565(25)11167-1. PMID 1602399.
- ^ a b c d Schweri MM, de Costa BR, Rice KC (July 1998). "Fourphit, an acylating phencyclidine derivative, attenuates cocaine-induced hyperactivity in rats". Pharmacol Biochem Behav. 60 (3): 615–623. doi:10.1016/s0091-3057(98)00040-9. PMID 9678644.
| AMPARTooltip α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor |
|
|---|---|
| KARTooltip Kainate receptor |
|
| NMDARTooltip N-Methyl-D-aspartate receptor |
|
| |
This article is issued from Wikipedia. The text is available under Creative Commons Attribution-Share Alike 4.0 unless otherwise noted. Additional terms may apply for the media files.