Levoketoconazole
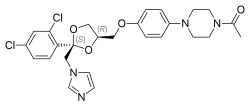 | |
| Clinical data | |
|---|---|
| Trade names | Recorlev |
| Other names | COR-003; (2S,4R)-ketoconazole; NormoCort |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C26H28Cl2N4O4 |
| Molar mass | 531.43 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Levoketoconazole, sold under the brand name Recorlev, is a steroidogenesis inhibitor that is used for the treatment of Cushing's syndrome.[2][3][4][5] Levoketoconazole was approved for medical use in the United States in December 2021.[6][7]
Levoketoconazole is the levorotatory or (2S,4R) enantiomer of ketoconazole,[3][4][5] and it is an inhibitor of the enzymes CYP11B1 (11β-hydroxylase), CYP17A1 (17α-hydroxylase/17,20-lyase), and CYP21A2 (21-hydroxylase).[2][3][5] It inhibits glucocorticoid biosynthesis and hence circulating levels of glucocorticoids, thereby treating Cushing's syndrome.[2][5] In addition to its increased potency, the drug is 12-fold less potent than racemic ketoconazole in inhibiting CYP7A1 (cholesterol 7α-hydroxylase), theoretically resulting in further reduced interference with bile acid production and metabolite elimination and therefore less risk of hepatotoxicity.[5] Levoketoconazole has also been found to inhibit CYP11A1 (cholesterol side-chain cleavage enzyme) and CYP51A1 (lanosterol-14α-demethylase), similarly but more potently relative to ketoconazole.[8]
Research
In a systematic review of levoketoconazole, published in 2024, it was found to be effective in the management of Cushing Syndrome.[9]
References
- ^ "Recorlev- levoketoconazole tablet". DailyMed. 12 January 2022. Retrieved 20 February 2022.
- ^ a b c "Levoketoconazole - Strongbridge Biopharma". AdisInsight. Springer Nature Switzerland AG.
- ^ a b c Laws Jr ER, Pace L (11 November 2016). Cushing's Disease: An Often Misdiagnosed and Not So Rare Disorder. Elsevier Science. pp. 113–. ISBN 978-0-12-804390-5.
- ^ a b Geer EB (1 December 2016). The Hypothalamic-Pituitary-Adrenal Axis in Health and Disease: Cushing's Syndrome and Beyond. Springer. pp. 170–. ISBN 978-3-319-45950-9.
- ^ a b c d e Cuevas-Ramos D, Lim DS, Fleseriu M (2016). "Update on medical treatment for Cushing's disease". Clinical Diabetes and Endocrinology. 2 (1) 16. doi:10.1186/s40842-016-0033-9. PMC 5471955. PMID 28702250.
- ^ "Levoketoconazole: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 3 January 2022. Retrieved 3 January 2022.
- ^ "Xeris Biopharma Announces U.S. FDA Approval of Recorlev (levoketoconazole) for the Treatment of Endogenous Hypercortisolemia in Adult Patients With Cushing's Syndrome" (Press release). Xeris Biopharma. 30 December 2021. Retrieved 3 January 2022 – via Business Wire.
- ^ Thieroff-Ekerdt R, Lavin P, Abou-Gharbia M, France N (October 2016). Pharmacology of COR-003 (levoketoconazole), an investigational treatment for endogenous Cushing's syndrome (PDF). Pituitary disorders—it’s not the anterior pituitary (posters). Endocrine Society. pp. SAT-547 – SAT-547. Archived from the original (PDF) on 20 September 2020. Retrieved 30 April 2017.
- ^ Patra S, Dutta D, Nagendra L, Raizada N (2024). "Efficacy and Safety of Levoketoconazole in Managing Cushing's Syndrome: A Systematic Review". Indian Journal of Endocrinology and Metabolism. 28 (4): 343–349. doi:10.4103/ijem.ijem_477_23. PMC 11451957. PMID 39371660.
External links
- Clinical trial number NCT03277690 for "A Study to Assess the Safety and Efficacy of Levoketoconazole in the Treatment of Endogenous Cushing's Syndrome" at ClinicalTrials.gov
- Clinical trial number NCT01838551 for "Treatment for Endogenous Cushing's Syndrome (SONICS)" at ClinicalTrials.gov