Dapiprazole
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a601043 |
| Pregnancy category |
|
| Routes of administration | Topical (eye drops) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Negligible when administered topically |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
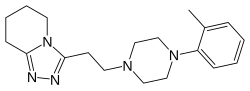
| Formula | C19H27N5 |
| Molar mass | 325.460 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Dapiprazole (brand name Rev-Eyes) is an alpha-1 blocker. It is used to reverse mydriasis after eye examination.[1]
References