Redafamdastat
 | |
| Clinical data | |
|---|---|
| Other names | JZP-150; JZP150; PF-04457845; PF-4457845; PF04457845; PF4457845 |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | CY3A4 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
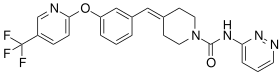
| Formula | C23H20F3N5O2 |
| Molar mass | 455.441 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Redafamdastat (INN; developmental code names JZP-150, PF-04457845) is an inhibitor of the enzyme fatty acid amide hydrolase (FAAH), with an IC50 of 7.2 nM, and both analgesic and anti-inflammatory effects in animal studies comparable to those of the cyclooxygenase inhibitor naproxen.[1] It was being developed by Jazz Pharmaceuticals for the treatment of alcoholism, pain, and post-traumatic stress disorder (PTSD) and reached phase 2 clinical trials.[2][3] However, development of the drug was discontinued in December 2023.[2]
See also
References
- ^ Johnson DS, Stiff C, Lazerwith SE, Kesten SR, Fay LK, Morris M, et al. (February 2011). "Discovery of PF-04457845: A Highly Potent, Orally Bioavailable, and Selective Urea FAAH Inhibitor". ACS Medicinal Chemistry Letters. 2 (2): 91–96. doi:10.1021/ml100190t. PMC 3109749. PMID 21666860.
- ^ a b "JZP 150". AdisInsight. 26 December 2023. Retrieved 16 August 2024.
- ^ "A Study of JZP150 in Adults With Posttraumatic Stress Disorder - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. 5 December 2024.