Endrisone
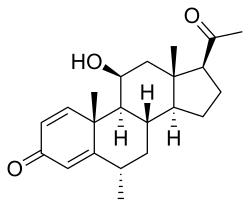 | |
| Clinical data | |
|---|---|
| Trade names | Aldrisone |
| Other names | Delta-medrysone;[1] 6α-Methyl-11β-hydroxypregna-1,4-diene-3,20-dione; 6α-Methyl-11β-hydroxy-Δ1-progesterone |
| Routes of administration | Topical, ophthalmic[1][2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.047.587 |
| Chemical and physical data | |
| Formula | C22H30O3 |
| Molar mass | 342.479 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Endrisone (INN; also known as endrysone (USAN); brand name Aldrisone) is a synthetic, steroidal glucocorticoid which is or has been marketed in Italy by SIFI.[1][2][3] It is used as a topical and ophthalmic anti-inflammatory drug in the treatment of skin and eye conditions, respectively.[1][2]
See also
References
- ^ a b c d Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 486–. ISBN 978-1-4757-2085-3.
- ^ a b c Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 110–. ISBN 978-94-011-4439-1.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 386–. ISBN 978-3-88763-075-1.