Halcinonide
 | |
| Clinical data | |
|---|---|
| Trade names | Halog |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a682272 |
| Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.019.490 |
| Chemical and physical data | |
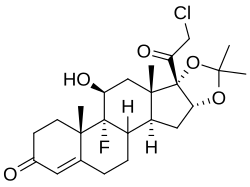
| Formula | C24H32ClFO5 |
| Molar mass | 454.96 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Halcinonide is a high potency corticosteroid, in group II (second most potent group) under US classification.[1] It is used topically (in a 0.05% cream provided as Halog) in the treatment of certain skin conditions. It is available as a generic medication.[2]
References
- ^ Pujos E, Flament-Waton MM, Paisse O, Grenier-Loustalot MF (January 2005). "Comparison of the analysis of corticosteroids using different techniques". Anal Bioanal Chem. 381 (1): 244–54. doi:10.1007/s00216-004-2890-9. PMID 15700162. S2CID 19561444.
- ^ "First Generic Drug Approvals". U.S. Food and Drug Administration (FDA). 8 July 2024. Retrieved 9 July 2024.